Abstract
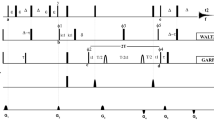
The limits of resolution that can be obtained in 1H–15N 2D NMR spectroscopy of isotopically enriched nanocrystalline proteins are explored. Combinations of frequency switched Lee–Goldburg (FSLG) decoupling, fast magic angle sample spinning (MAS), and isotopic dilution via deuteration are investigated as methods for narrowing the amide 1H resonances. Heteronuclear decoupling of 15N from the 1H resonances is also studied. Using human ubiquitin as a model system, the best resolution is most easily obtained with uniformly 2H and 15N enriched protein where the amides have been exchanged in normal water, MAS at ∼20 kHz, and WALTZ-16 decoupling of the 15N nuclei. The combination of these techniques results in average 1H lines of only ∼0.26 ppm full width at half maximum. Techniques for optimizing instrument stability and 15N decoupling are described for achieving the best possible performance in these experiments.
Similar content being viewed by others
References
A.E. Bennett C.M. Rienstra M. Auger K.V. Lakshmi R.G. Griffin (1995) J. Chem. Phys. 103 6951–6958
A. Bielecki A.C. Kolbert M.H. Levitt (1989) Chem. Phys. Lett. 155 341–346
C.E. Bronnimann B.L. Hawkins M. Zhang G.E. Maciel (1988) Anal. Chem. 60 1743–1750
V. Chevelkov B.J. Rossum F. Castellani K. Rehbein A. Diehl M. Hohwy S. Steuernagel F. Engelke H. Oschkinat B. Reif (2003) J. Am. Chem. Soc. 125 7788–7789
A. Detken E.H. Hardy M. Ernst B.H. Meier (2002) Chem. Phys. Lett. 356 298–304
M. Ernst S. Bush A.C. Kolbert A. Pines (1996) J. Chem. Phys. 105 3387–3397
T. Gullion D.B. Baker M.S. Conradi (1990) J. Magn. Reson. 89 479–484
A.W. Hing S. Vega J. Schaefer (1992) J. Magn. Reson. 96 205–209
Igumenova, T.I. (2003) Assignment of Uniformly 13C Enriched Proteins and Optimization of Their Lineshapes, Ph.D. Thesis, Columbia, New York
Y. Ishii J. Ashida T. Terao (1995) Chem. Phys. Lett. 246 439–445
H. Kimura K. Nakamura A. Eguchi H. Sugisawa K. Deguchi K. Ebisawa E. Suzuki A. Shoji (1998) J. Mol. Struct. 447 247–255
A. Lesage L. Emsley (2001) J. Magn. Reson. 148 449–454
J.L. Markley A. Bax Y. Arata C.W. Hilbers R. Kaptein B.D. Sykes P.E. Wright K. Wuthrich (1998) Pure Appl. Chem. 70 117–142
R.W. Martin E.K. Paulson K.W. Zilm (2003) Rev. Sci. Instrum. 74 3045–3061
R.W. Martin K.W. Zilm (2003) J. Magn. Reson. 165 162–174 Occurrence Handle10.1016/S1090-7807(03)00253-2
R.W. Martin K.W. Zilm (2004) J. Magn. Reson. 168 202–209
A.E. McDermott F.J. Creuzet A.C. Kolbert R.G. Griffin (1992) J. Magn. Reson. 98 408–413
Morcombe, C.R. (2004) Solid State Nuclear Magnetic Resonance of Deuterated Ubiquitin, Ph.D. Thesis, Yale University
C.R. Morcombe K.W. Zilm (2003) J. Magn. Reson. 162 479–486
S. Neal A.M. Nip H.Y. Zhang D.S. Wishart (2003) J. Biomol. NMR 26 215–240
E.T. Olejniczak S. Vega R.G. Griffin (1984) J. Chem. Phys. 81 4804–4817
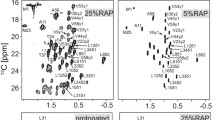
E.K. Paulson C.R. Morcombe V. Gaponenko B. Dancheck R.A. Byrd K.W. Zilm (2003) J. Am. Chem. Soc. 125 15831–15836
B. Reif C.P. Jaroniec C.M. Rienstra M. Hohwy R.G. Griffin (2001) J. Magn. Reson. 151 320–327
B. Reif B.J. Rossum Particlevan F. Castellani K. Rehbein A. Diehl H. Oschkinat (2003) J. Am. Chem. Soc. 125 1488–1489
W.K. Rhim D.D. Elleman Lb. Schreibe R.W. Vaughan (1974) J. Chem. Phys. 60 4595–4604
W.K. Rhim D.D. Elleman R.W. Vaughan (1972) Bull. Am. Phys. Soc. 17 1182–1182
W.K. Rhim D.D. Elleman R.W. Vaughan (1973) J. Chem. Phys. 59 3740–3749
J.E. Roberts S. Vega R.G. Griffin (1984) J. Am. Chem. Soc. 106 2506–2512
L.M. Ryan R.E. Taylor A.J. Paff B.C. Gerstein (1980) J. Chem. Phys. 72 508–515
I. Schnell B. Langer S.H.M. Sontjens Particlevan M.H.P. Genderen R.P. Sijbesma H.W. Spiess (2001) J. Magn. Reson. 150 57–70
B.J. vanRossum F. Castellani J. Pauli K. Rehbein J. Hollander Particlede H.J.M. Groot H. Oschkinat (2003) J. Biomol. NMR 25 217–223
B.J. vanRossum H. Forster H.J.M. Groot Particlede (1997) J. Magn. Reson. 124 516–519
A.J. Vega (2004) J. Magn. Reson. 170 22–41
E. Vinogradov P.K. Madhu S. Vega (1999) Chem. Phys. Lett. 314 443–450
E. Vinogradov P.K. Madhu S. Vega (2001) J. Chem. Phys. 115 8983–9000
J.S. Waugh L.M. Huber U. Haeberle (1968) Phys. Rev. Lett. 20 180–182
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morcombe, C.R., Paulson, E.K., Gaponenko, V. et al. 1H–15N correlation spectroscopy of nanocrystalline proteins. J Biomol NMR 31, 217–230 (2005). https://doi.org/10.1007/s10858-005-0527-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10858-005-0527-8