Abstract
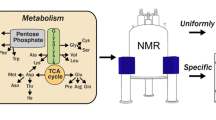
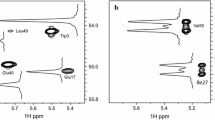
A labeling scheme is introduced that facilitates the measurement of accurate 13Cβ chemical shifts of invisible, excited states of proteins by relaxation dispersion NMR spectroscopy. The approach makes use of protein over-expression in a strain of E. coli in which the TCA cycle enzyme succinate dehydrogenase is knocked out, leading to the production of samples with high levels of 13C enrichment (30–40%) at Cβ side-chain carbon positions for 15 of the amino acids with little 13C label at positions one bond removed (≈5%). A pair of samples are produced using [1-13C]-glucose/NaH12CO3 or [2-13C]-glucose as carbon sources with isolated and enriched (>30%) 13Cβ positions for 11 and 4 residues, respectively. The efficacy of the labeling procedure is established by NMR spectroscopy. The utility of such samples for measurement of 13Cβ chemical shifts of invisible, excited states in exchange with visible, ground conformations is confirmed by relaxation dispersion studies of a protein–ligand binding exchange reaction in which the extracted chemical shift differences from dispersion profiles compare favorably with those obtained directly from measurements on ligand free and fully bound protein samples.







Similar content being viewed by others
References
Boehr DD, McElheny D, Dyson HJ, Wright PE (2006) The dynamic energy landscape of dihydrofolate reductase catalysis. Science 313:1638–1642
Bystrov VF (1976) Spin-spin coupling and the conformational states of peptide systems. Prog Nucl Mag Res Spectrosc 10:41–82
Carr HY, Purcell EM (1954) Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys Rev 94:630–638
Castellani F, van Rossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H (2002) Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature 420:98–102
Cornilescu G, Delaglio F, Bax A (1999) Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR 13:289–302
Cronan JE, LaPorte DC (1996) Tricarboxylic cycle and glyoxylate bypass. In: Neidhardt FC, Curtiss R (eds) Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington D.C., pp 206–216
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe—a multidimensional spectral processing system based on unix pipes. J Biomol NMR 6:277–293
Drubin DG, Mulholland J, Zhu ZM, Botstein D (1990) Homology of a yeast actin-binding protein to signal transduction proteins and myosin-I. Nature 343:288–290
Eisenmesser EZ, Bosco DA, Akke M, Kern D (2002) Enzyme dynamics during catalysis. Science 295:1520–1523
Eisenmesser EZ, Millet O, Labeikovsky W, Korzhnev DM, Wolf-Watz M, Bosco DA, Skalicky JJ, Kay LE, Kern D (2005) Intrinsic dynamics of an enzyme underlies catalysis. Nature 438:117–121
Farmer BT, Venters RA (1999) NMR of perdeuterated larger proteins. In: Krishna NR, Berliner LJ (eds) Biological magnetic resonance, vol 16. Kluwer Academic/Plenum Publishers, New York, pp 75–120
Freeman R (1999) Spin choreography—basic steps in high resolution NMR. Oxford University Press, Oxford, p 123
Gardner KH, Kay LE (1998) The use of 2H, 13C, 15N multidimensional NMR to study the structure and dynamics of proteins. Annu Rev Biophys Biomol Struct 27:357–406
Geen H, Freeman R (1991) Band-selective radiofrequency pulses. J Magn Reson 93:93–141
Goto NK, Gardner KH, Mueller GA, Willis RC, Kay LE (1999) A robust and cost-effective method for the production of Val, Leu, Ile (δ1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J Biomol NMR 13:369–374
Gronostajski RM, Sadowski PD (1985) The Flp protein of the 2-micron plasmid of yeast—intermolecular and intramolecular reactions. J Biol Chem 260:2328–2335
Gross JD, Gelev VM, Wagner G (2003) A sensitive and robust method for obtaining intermolecular NOEs between side chains in large protein complexes. J Biomol NMR 25:235–242
Grzesiek S, Anglister J, Ren H, Bax A (1993) 13C line narrowing by 2H decoupling in 2H/13C/15N-enriched proteins—application to triple-resonance 4D J-connectivity of sequential amides. J Am Chem Soc 115:4369–4370
Hahn EL, Maxwell DE (1952) Spin echo measurements of nuclear spin coupling in molecules. Phys Rev 88:1070–1084
Hansen DF, Vallurupalli P, Kay LE (2008a) Quantifying two-bond 1HN-13CO and one-bond 1Hα-13Cα dipolar couplings of invisible protein states by spin-state selective relaxation dispersion NMR spectroscopy. J Am Chem Soc 130:8397–8405
Hansen DF, Vallurupalli P, Lundström P, Neudecker P, Kay LE (2008b) Probing chemical shifts of invisible states of proteins with relaxation dispersion NMR spectroscopy: how well can we do? J Am Chem Soc 130:2667–2675
Haynes J, Garcia B, Stollar EJ, Rath A, Andrews BJ, Davidson AR (2007) The biologically relevant targets and binding affinity requirements for the function of the yeast actin-binding protein 1 Src-homology 3 domain vary with genetic context. Genetics 176:193–208
Hill RB, Bracken C, DeGrado WF, Palmer AG (2000) Molecular motions and protein folding: characterization of the backbone dynamics and folding equilibrium of α2D using 13C NMR spin relaxation. J Am Chem Soc 122:11610–11619
Igumenova TI, Brath U, Akke M, Palmer AG (2007) Characterization of chemical exchange using residual dipolar coupling. J Am Chem Soc 129:13396–13397
Ikura M, Bax A (1992) Isotope-filtered 2D NMR of a protein peptide complex—study of a skeletal-muscle myosin light chain kinase fragment bound to calmodulin. J Am Chem Soc 114:2433–2440
Ishima R, Torchia DA (2003) Extending the range of amide proton relaxation dispersion experiments in proteins using a constant-time relaxation-compensated CPMG approach. J Biomol NMR 25:243–248
Ishima R, Baber J, Louis JM, Torchia DA (2004) Carbonyl carbon transverse relaxation dispersion measurements and ms-μs timescale motion in a protein hydrogen bond network. J Biomol NMR 29:187–198
Kainosho M, Torizawa T, Iwashita Y, Terauchi T, Ono AM, Guntert P (2006) Optimal isotope labelling for NMR protein structure determinations. Nature 440:52–57
Kay LE, Torchia DA, Bax A (1989) Backbone dynamics of proteins as studied by 15N inverse detected heteronuclear NMR-spectroscopy—application to staphylococcal nuclease. Biochemistry 28:8972–8979
Kay LE, Clore GM, Bax A, Gronenborn AM (1990a) 4-Dimensional heteronuclear triple-resonance NMR spectroscopy of interleukin-1-beta in solution. Science 249:411–414
Kay LE, Ikura M, Tschudin R, Bax A (1990b) 3-Dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson 89:496–514
Korzhnev DM, Salvatella X, Vendruscolo M, Di Nardo AA, Davidson AR, Dobson CM, Kay LE (2004) Low-populated folding intermediates of Fyn SH3 characterized by relaxation dispersion NMR. Nature 430:586–590
Korzhnev DM, Neudecker P, Mittermaier A, Orekhov VY, Kay LE (2005) Multiple-site exchange in proteins studied with a suite of six NMR relaxation dispersion experiments: an application to the folding of a Fyn SH3 domain mutant. J Am Chem Soc 127:15602–15611
Lee LK, Rance M, Chazin WJ, Palmer AG (1997) Rotational diffusion anisotropy of proteins from simultaneous analysis of N-15 and C-13(alpha) nuclear spin relaxation. J Biomol NMR 9:287–298
LeMaster DM (1990) Deuterium labeling in NMR structural analysis of larger proteins. Q Rev Biophys 23:133–174
LeMaster DM, Kushlan DM (1996) Dynamical mapping of E. coli thioredoxin via 13C NMR relaxation analysis. J Am Chem Soc 118:9255–9264
Levitt MH (1986) Composite pulses. Prog Nucl Mag Res Spectrosc 18:61–122
Li M, Ho PY, Yao SJ, Shimizu K (2006) Effect of sucA or sucC gene knockout on the metabolism in Escherichia coli based on gene expressions, enzyme activities, intracellular metabolite concentrations and metabolic fluxes by C-13-labeling experiments. Biochem Eng J 30:286–296
Lila T, Drubin DG (1997) Evidence for physical and functional interactions among two Saccharomyces cerevisiae SH3 domain proteins, an adenylyl cyclase-associated protein and the actin cytoskeleton. Mol Biol Cell 8:367–385
Löhr F, Rüterjans H (1998) Detection of nitrogen-nitrogen J-couplings in proteins. J Magn Reson 132:130–137
Loria JP, Rance M, Palmer AG (1999) A relaxation-compensated Carr-Purcell-Meiboom-Gill sequence for characterizing chemical exchange by NMR spectroscopy. J Am Chem Soc 121:2331–2332
Lundström P, Teilum K, Carstensen T, Bezsonova I, Wiesner S, Hansen DF, Religa TL, Akke M, Kay LE (2007a) Fractional 13C enrichment of isolated carbons using [1–13C]- or [2–13C]-glucose facilitates the accurate measurement of dynamics at backbone Ca and side-chain methyl positions in proteins. J Biomol NMR 38:199–212
Lundström P, Vallurupalli P, Religa TL, Dahlquist FW, Kay LE (2007b) A single-quantum methyl 13C-relaxation dispersion experiment with improved sensitivity. J Biomol NMR 38:79–88
Lundström P, Hansen DF, Kay LE (2008) Measurement of carbonyl chemical shifts of excited protein states by relaxation dispersion NMR spectroscopy: comparison between uniformly and selectively 13C labeled samples. J Biomol NMR 42:35–47
Lundström P, Hansen DF, Vallurupalli P, Kay LE (2009) Accurate measurement of alpha proton chemical shifts of excited protein states by relaxation dispersion NMR spectroscopy. J Am Chem Soc 131:1915–1926
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR-spectra without phase cycling—application to the study of hydrogen-exchange in proteins. J Magn Reson 85:393–399
Meiboom S, Gill D (1958) Modified spin-echo method for measuring nuclear relaxation times. Rev Sci Instrum 29:688–691
Molenaar D, Van der Rest ME, Petrovic S (1998) Biochemical and genetic characterization of the membrane-associated malate dehydrogenase (acceptor) from Corynebacterium glutamicum. Eur J Biochem 254:395–403
Montelione GT, Wagner G (1990) Conformation-independent sequential NMR connections in isotope-enriched polypeptides by 1H–13C–15N triple-resonance experiments. J Magn Reson 87:183–188
Mulder FAA, Mittermaier A, Hon B, Dahlquist FW, Kay LE (2001a) Studying excited states of proteins by NMR spectroscopy. Nat Struct Biol 8:932–935
Mulder FAA, Skrynnikov NR, Hon B, Dahlquist FW, Kay LE (2001b) Measurement of slow (μs-ms) time scale dynamics in protein side chains by 15N relaxation dispersion NMR spectroscopy: application to Asn and Gln residues in a cavity mutant of T4 lysozyme. J Am Chem Soc 123:967–975
Mulder FAA, Hon B, Mittermaier A, Dahlquist FW, Kay LE (2002) Slow internal dynamics in proteins: application of NMR relaxation dispersion spectroscopy to methyl groups in a cavity mutant of T4 lysozyme. J Am Chem Soc 124:1443–1451
Palmer AG, Kroenke CD, Loria JP (2001) Nuclear magnetic resonance methods for quantifying microsecond-to-millisecond motions in biological macromolecules. Methods Enzymol 339:204–238
Palmer AG, Grey MJ, Wang CY (2005) Solution NMR spin relaxation methods for characterizing chemical exchange in high-molecular-weight systems. Methods Enzymol 394:430–465
Press WH, Flannery BP, Teukolsky SA and Vetterling WT (1988) Numerical recipes in C. University Press, Cambridge
Rath A, Davidson AR (2000) The design of a hyperstable mutant of the Abp1p SH3 domain by sequence alignment analysis. Protein Sci 9:2457–2469
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Mag Res Spectrosc 34:93–158
Shaka AJ, Barker PB, Freeman R (1985) Computer-optimized decoupling scheme for wideband applications and low-level operation. J Magn Reson 64:547–552
Shen Y, Bax A (2007) Protein backbone chemical shifts predicted from searching a database for torsion angle and sequence homology. J Biomol NMR 38:289–302
Skrynnikov NR, Dahlquist FW, Kay LE (2002) Reconstructing NMR spectra of “invisible” excited protein states using HSQC and HMQC experiments. J Am Chem Soc 124:12352–12360
Spera S, Bax A (1991) Empirical correlation between protein backbone conformation and Cα and Cβ–13C nuclear magnetic resonance chemical shifts. J Am Chem Soc 113:5490–5492
Studier FW, Moffatt BA (1986) Use of bacteriophage-T7 RNA-polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130
Sugase K, Dyson HJ, Wright PE (2007) Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 447:1021–1025
Tollinger M, Skrynnikov NR, Mulder FAA, Forman-Kay JD, Kay LE (2001) Slow dynamics in folded and unfolded states of an SH3 domain. J Am Chem Soc 123:11341–11352
Tugarinov V, Kay LE (2004) An isotope labeling strategy for methyl TROSY spectroscopy. J Biomol NMR 28:165–172
Vallurupalli P, Kay LE (2006) Complementarity of ensemble and single-molecule measures of protein motion: a relaxation dispersion NMR study of an enzyme complex. Proc Natl Acad Sci USA 103:11910–11915
Vallurupalli P, Hansen DF, Stollar E, Meirovitch E, Kay LE (2007) Measurement of bond vector orientations in invisible excited states of proteins. Proc Natl Acad Sci USA 104:18473–18477
Vallurupalli P, Hansen DF, Kay LE (2008a) Probing structure in invisible protein states with anisotropic NMR chemical shifts. J Am Chem Soc 130:2734–2735
Vallurupalli P, Hansen DF, Kay LE (2008b) Structures of invisible, excited protein states by relaxation dispersion NMR spectroscopy. Proc Natl Acad Sci USA 105:11766–11771
Vallurupalli P, Hansen DF, Lundström P, Kay LE (2009) CPMG relaxation dispersion NMR experiments measuring glycine 1Hα and 13Cα chemical shifts in the ‘invisible’ excited states of proteins. J Biomol NMR. doi 10.1007/s10858-009-9310-6
Voet D, Voet JG (1995) Biochemistry. Wiley, Hoboken
Wand AJ, Bieber RJ, Urbauer JL, McEvoy RP, Gan ZH (1995) Carbon relaxation in randomly fractionally 13C-enriched proteins. J Magn Reson B 108:173–175
Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH (2008) Amyloid fibrils of the HET-s (218–289) prion form a beta solenoid with a triangular hydrophobic core. Science 319:1523–1526
Watt ED, Shimada H, Kovrigin EL, Loria JP (2007) The mechanism of rate-limiting motions in enzyme function. Proc Natl Acad Sci USA 104:11981–11986
Wishart DS, Case DA (2002) Use of chemical shifts in macromolecular structure determination. Methods Enzymol 338:3–34
Wishart DS, Sykes BD (1994) The 13C chemical-shift index—a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR 4:171–180
Wolf-Watz M, Thai V, Henzler-Wildman K, Hadjipavlou G, Eisenmesser EZ, Kern D (2004) Linkage between dynamics and catalysis in a thermophilic-mesophilic enzyme pair. Nat Struct Mol Biol 11:945–949
Zeeb M, Balbach J (2005) NMR spectroscopic characterization of millisecond protein folding by transverse relaxation dispersion measurements. J Am Chem Soc 127:13207–13212
Zuiderweg ERP, Petros AM, Fesik SW, Olejniczak ET (1991) 4-Dimensional [13C, 1H, 13C, 1H] HMQC-NOE-HMQC NMR-spectroscopy—resolving tertiary NOE distance constraints in the spectra of larger proteins. J Am Chem Soc 113:370–372
Acknowledgments
We thank Dr. Jack Greenblatt, University of Toronto, for the gift of plasmids used for the knockout of succinate dehydrogenase and for valuable advice regarding this procedure. P. Vallurupalli and D. F. Hansen are thanked for useful discussion. This research was supported by a grant from The Canadian Institutes of Health Research. PL is supported by The Swedish Research Council. LEK holds a Canadian Research Chair in Biochemistry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lundström, P., Lin, H. & Kay, L.E. Measuring 13Cβ chemical shifts of invisible excited states in proteins by relaxation dispersion NMR spectroscopy. J Biomol NMR 44, 139–155 (2009). https://doi.org/10.1007/s10858-009-9321-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-009-9321-3