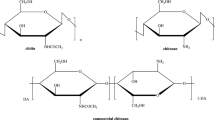
Abstract
Chitin biopolymer production and its by-product chitosan show great potential. These biomaterials have great applicability in various fields because they are non-toxic, biodegradable, biocompatible, and have antimicrobial effects. The most common source of chitin and chitosan is the crustaceous shell; however, mushrooms are an alternative source for isolating these biopolymers because their cellular wall has a high content of chitin, which may be transformed into chitosan through a deacetylation reaction. The main objective of this research was to obtain chitosan through the deacetylation of chitin isolated from the Ganoderma lucidum basidiomycetes mushroom, which is obtained through biotechnological culture. The material characterization was performed using X-ray diffraction, Fourier transform infrared spectroscopy, thermogravimetric analysis, and an evaluation of cytotoxicity comparing the results obtained with results for commercial chitosan. Protocol results showed that chitosan obtained from this mushroom had a significant similitude with commercial chitosan, yet the one obtained using P2 protocol was the one that rendered the best results: including diffractogram peaks, characteristic infrared analysis bands, and an 80.29 % degree of deacetylation. Cytotoxicity in vitro testing showed that the material was non-toxic; furthermore, it rendered very promising information regarding the evaluation of future applications of this biomaterial in the field of biomedicine.




Similar content being viewed by others
References
Wan ACA, Tai BCU. CHITIN—A promising biomaterial for tissue engineering and stem cell technologies. Biotechnol Adv. 2013;31(8):1776–85.
Dutta PK, Ravikumar MNV, Dutta J. Chitin and chitosan for versatile applications. Polym Rev. 2002;42(3):307–54.
Muzzarelli RAA. Jeuniaux C. In: Muzzarelli RAA, editor. Chitin. New York: Pergamon Press; 1976.
Khor E. Chitin: fulfilling a biomaterials promise. Amsterdam: Elsevier Applied Science; 2001.
Bough WA, Satter WL, Wu AC, Perkin BE. Influence of manufacturing variables on the characteristics and effectiveness of chitin products I: chemical compositions, viscosity and molecular weight distribution of chitosan products. Biotechnol Bioeng. 1978;20:1931–43.
Chandumpai A, Singhpibulporn N, Faroongsarng D, Sornprasit P. Preparation and physico-chemical characterization of chitin and chitosan from the pens of the squid species, Loligo lessoniana and Loligo formosana. Carbohydr Polym. 2004;58(4):467–74.
Caprile MD. Obtención y utilización de quitina y quitosano a partir de desechos de crustáceos. Congreso Mundial ISWA 2005: “Hacia un sistema integral de residuos sólidos urbanos”. Argentina.
Ospina SP, Ramírez DA, Escobar DM, Ossa CPO, Rojas D, Atehortúa L, Zapata P. Comparison of extraction methods chitin from Ganoderma lucidum Mushroom obtained in submerged culture. Biomed Res Int. 2014;2014:1–7.
Musarrat HM, Williams PA, Tverezovskaya O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013;31(2):166–71.
Zapata P, Rojas D, Atehortua L. Production of biomass, polysaccharides and ganoderic acid using non-conventional carbon sources under submerged culture of the Ganoderma lucidum (W.Curt.:Fr.)P. Karst. (higher Basidiomycetes). Int J Med Mushrooms. 2012;14(2):197–203.
Rojas D, Zapata P, Palacio A, Ospina S, Atehortúa L. Basidiomycetes Mushroom Biotechnology for the Development of Functional Products: the Effect of Drying Processes on Biological Activity. Open Conf Proc J. 2013;4:93–8.
Peniche Covas C. Estudios sobre quitina y quitosana. Tesis Doctoral, Facultad de Química, Universidad de La Habana, Cuba; 2006.
Di Mario F, Rapanà P, Tomati U, Galli E. Chitin and chitosan from Basidiomycetes. Int J Biol Macromol. 2008;43(1):8–12.
Methacanon P, Prasitsilp M, Pothsree T, Pattaraarchachai J. Heterogeneous N-deacetylation of squid chitin in alkaline solution. Carbohydr Polym. 2003;52(2):119–23.
Brugnerotto J, Lizadi J, Goycoolea FM, Argüelles-Monal W, Desbrières J, Rinaudo M. An infrared investigation in relation with chitin and chitosan characterization. Polym. 2001;42:3569–80.
Chapter 87, Biological reactivity tests in vitro. United States Pharmacopoeia (USP29).
Galia CR, Macedo CA, Rosito R. Muller de Mello T, Araújo Quaresma Camargo L M, MoreiraI LF. In vitro and in vivo evaluation of lyophilized bovine bone biocompatibility. Clinics. 2008;63(6):801–6.
Pochanavanich P, Suntornsuk W. Fungal chitosan production and its characterization. Lett Appl Microbiol. 2002;35:17–21.
Olvera Acosta P. Aislamiento de levaduras que tengan la capacidad para degradar lignina y búsqueda de algunos genes implicados en dicha degradación. México; 2003. p. 79.
Quiriz FA. Evaluación de las propiedades antioxidantes y antimicrobianas de dos especies del hongo medicinal Ganoderma nativo de México y su contribución al desarrollo regional. México; 2012. p. 86.
Fernández Cervera M, Heinämäkib J, Räsänen M, Maunu SL, Karjalainen M, Nieto Acosta OM, Iraizoz Colarte A, Yliruusi J. Solid-state characterization of chitosans derived from lobster chitin. Carbohydr Polym. 2004;58(4):401–8.
Kasaai MR. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr Polym. 2008;71(4):497–508.
Laréz C. Quitina y quitosano: materiales del pasado para el presente y el futuro. Avances en Química. 2006;1(2):15–21.
Wu T, Zivanovic S, Draughon FA, Conway WS, Sams CE. Physicochemical Properties and Bioactivity of Fungal Chitin and Chitosan. J Agric Food Chem. 2005;53(10):3888–94.
Crestini C, Kovac B, Giovannozzi-Sermanni G. Production and isolation of chitosan by submerged and solid-state fermentation from Lentinus edodes. Biotechnology Bioengineering. 1996;50:207–10.
Ramya R, Sudha PN, Mahalakshmi J. Preparation and characterization of chitosan binary blend. Int. J. Sci. Res. Publ. 2012;2(10):1–9.
Přichystalová H, Almonasy N, Abdel-Mohsen AM, Abdel-Rahmana RM, Fouda MM, Vojtova L, Kobera L, Spotz Z, Burgert L, Jancar J. Synthesis, characterization and antibacterial activity of new fluorescent chitosan derivatives. Int J Biol Macromol. 2014;65:234–40.
Paulino AT, Simionato JI, Garcia JC, Nozaki J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr Polym. 2006;64(1):98–103.
Kaya M, Baran T, Erdoğan S, Mentes A, Özüsağlam MA, Çakmak YS. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater Sci Eng, C. 2014;45:72–81.
Zakaria Z, Izzah Z, Jawaid M, Hassan A. Effect of degree of deacetylation of chitosan on thermal stability and compatibility of chitosan-polyamide blend. Bioresourses. 2012;7(4):5568–80.
Liao SK, Hung CC, Lin MF. A kinetic study of thermal degradations of chitosan/polycaprolactam blends. Macromol Res. 2004;12(5):466–73.
Ivshin VP, Artamonova SD, Ivshina TN, Sharnina FF. Methods for isolation of chitin–glucan complexes from higher fungi native biomass. Polym Sci. 2007;49:2215–22.
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100(1):5–28.
Elsabee MZ, Abdou ES. Chitosan based edible films and coatings: a review. Mater Sci Eng C Mater Biol Appl. 2013;33(4):1819–41.
Gol NB, Patel PR, Rao TVR. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol Technol. 2013;85:185–95.
Hong K, Xie J, Zhang L, Sun D, Gong D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci Hortic. 2012;144:172–8.
Acknowledgments
The authors of this study express their gratitude to both Universidad de Antioquia CODI for its funding of this research project and to the Biotechnology and Biomaterial Research Groups.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mesa Ospina, N., Ospina Alvarez, S.P., Escobar Sierra, D.M. et al. Isolation of chitosan from Ganoderma lucidum mushroom for biomedical applications. J Mater Sci: Mater Med 26, 135 (2015). https://doi.org/10.1007/s10856-015-5461-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5461-z