Abstract
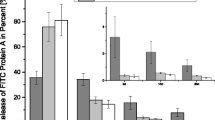
Bone morphogenetic proteins (BMPs) are the most potent osteoinductive growth factors. However, a delivery system is essential to take advantage of the osteoinductive effect of BMPs. The purpose of this study was to develop a sustained delivery system for recombinant human bone morphogenetic protein-2 (BMP-2). We covalently attached heparin to a cross-linked collagen type I coated tricalciumphosphate/hydroxyapatite (TCP/HA) bone substitute and subsequently loaded it with BMP-2. To systematically evaluate the contribution of each component with respect to the binding and release of BMP-2, six constructs were prepared and characterized: TCP/HA, TCP/HA with collagen (TCP/HACol), and TCP/HA with collagen and heparin (TCP/HAColHep) with and without BMP-2 (B). More BMP-2 bound to the TCP/HAColHep + B (92.9 ± 4.8 ng BMP-2/mg granule) granules as compared to the TCP/HACol + B (69.0 ± 9.6 ng BMP-2/mg granule) and TCP/HA + B granules (62.9 ± 5.4 ng BMP-2/mg granule). No difference in release pattern was found between the TCP/HA + B and TCP/HACol + B granules. Up to day 14, BMP-2 was still bound to the TCP/HAColHep + B granules, whereas most BMP had been released from TCP/HACol + B and TCP/HA + B granules at that time. After 21 days most BMP-2 also had been released from the TCP/HAColHep + B granules. The local and sustained delivery system for BMP-2 developed in this study may be useful as a carrier for BMP-2 and could possibly enhance bone regeneration efficacy for the treatment of large bone defects.





Similar content being viewed by others
References
Giannoudis PV. Chris Arts JJ, Schmidmaier G, et al. What should be the characteristics of the ideal bone graft substitute? Injury. 2011;42(Suppl 2):S1–2.
Larsson S, Hannink G. Injectable bone-graft substitutes: current products, their characteristics and indications, and new developments. Injury. 2011;42(Suppl 2):S30–4.
LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002;395(395):81–98.
LeGeros RZ, Lin S, Rohanizadeh R, et al. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med. 2003;14(3):201–9.
Hannink G, Arts JJ. Bioresorbability, porosity and mechanical strength of bone substitutes: what is optimal for bone regeneration? Injury. 2011;42(Suppl 2):S22–5.
Takahashi Y, Yamamoto M, Tabata Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials. 2005;26(23):4856–65.
Helm GA, Sheehan JM, Sheehan JP, et al. Utilization of type I collagen gel, demineralized bone matrix, and bone morphogenetic protein-2 to enhance autologous bone lumbar spinal fusion. J Neurosurg. 1997;86(1):93–100.
Yang XB, Whitaker MJ, Sebald W, et al. Human osteoprogenitor bone formation using encapsulated bone morphogenetic protein 2 in porous polymer scaffolds. Tissue Eng. 2004;10(7–8):1037–45.
Arnander C, Westermark A, Veltheim R, et al. Three-dimensional technology and bone morphogenetic protein in frontal bone reconstruction. J Craniofac Surg. 2006;17(2):275–9.
Noshi T, Yoshikawa T, Ikeuchi M, et al. Enhancement of the in vivo osteogenic potential of marrow/hydroxyapatite composites by bovine bone morphogenetic protein. J Biomed Mater Res. 2000;52(4):621–30.
Jeon O, Song SJ, Kang SW, et al. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28(17):2763–71.
Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: current challenges in BMP delivery. Biotechnol Lett. 2009;31(12):1817–24.
Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009;31(12):1825–35.
Rose FR, Hou Q, Oreffo RO. Delivery systems for bone growth factors—the new players in skeletal regeneration. J Pharm Pharmacol. 2004;56(4):415–27.
Nillesen ST, Geutjes PJ, Wismans R, et al. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28(6):1123–31.
Wissink MJ, Beernink R, Pieper JS, et al. Binding and release of basic fibroblast growth factor from heparinized collagen matrices. Biomaterials. 2001;22(16):2291–9.
Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237(1):295–302.
Pieper JS, Hafmans T, van Wachem PB, et al. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;62(2):185–94.
Sommer A, Rifkin DB. Interaction of heparin with human basic fibroblast growth factor: protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. J Cell Physiol. 1989;138(1):215–20.
Gospodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986;128(3):475–84.
Zhao B, Katagiri T, Toyoda H, et al. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem. 2006;281(32):23246–53.
Jackson RA, McDonald MM, Nurcombe V, et al. The use of heparan sulfate to augment fracture repair in a rat fracture model. J Orthop Res. 2006;24(4):636–44.
Blanquaert F, Saffar JL, Colombier ML, et al. Heparan-like molecules induce the repair of skull defects. Bone. 1995;17(6):499–506.
Pieper JS, Oosterhof A, Dijkstra PJ, et al. Preparation and characterization of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials. 1999;20(9):847–58.
Pieper JS, Hafmans T, Veerkamp JH, et al. Development of tailor-made collagen-glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials. 2000;21(6):581–93.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5.
Jeon O, Ryu SH, Chung JH, et al. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005;105(3):249–59.
Liu LS, Ng CK, Thompson AY, et al. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2). J Biomed Mater Res. 2002;62(1):128–35.
Perets A, Baruch Y, Weisbuch F, et al. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res A. 2003;65(4):489–97.
King GN. The importance of drug delivery to optimize the effects of bone morphogenetic proteins during periodontal regeneration. Curr Pharm Biotechnol. 2001;2(2):131–42.
den Boer FC, Wippermann BW, Blokhuis TJ, et al. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-1 or autologous bone marrow. J Orthop Res. 2003;21(3):521–8.
Winn SR, Uludag H, Hollinger JO. Carrier systems for bone morphogenetic proteins. Clin Orthop Relat Res Suppl. 1999;367(367 Suppl):95–106.
Alam MI, Asahina I, Ohmamiuda K, et al. Evaluation of ceramics composed of different hydroxyapatite to tricalcium phosphate ratios as carriers for rhBMP-2. Biomaterials. 2001;22(12):1643–51.
Arts JJ, Gardeniers JW, Welten ML, et al. No negative effects of bone impaction grafting with bone and ceramic mixtures. Clin Orthop. 2005;438:239–47.
Chow LC. Next generation calcium phosphate-based biomaterials. Dent Mater J. 2009;28(1):1–10.
Brodie JC, Goldie E, Connel G, et al. Osteoblast interactions with calcium phosphate ceramics modified by coating with type I collagen. J Biomed Mater Res A. 2005;73(4):409–21.
Weadock K, Olson RM, Silver FH. Evaluation of collagen crosslinking techniques. Biomater Med Devices Artif Organs. 1983;11(4):293–318.
Takada T, Katagiri T, Ifuku M, et al. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278(44):43229–35.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gerjon Hannink, Paul J. Geutjes contributed equally to the study.
Rights and permissions
About this article
Cite this article
Hannink, G., Geutjes, P.J., Daamen, W.F. et al. Evaluation of collagen/heparin coated TCP/HA granules for long-term delivery of BMP-2. J Mater Sci: Mater Med 24, 325–332 (2013). https://doi.org/10.1007/s10856-012-4802-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4802-4