Abstract
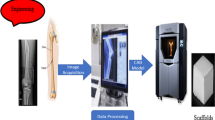
Three dimensional tissue engineered scaffolds for the treatment of critical defect have been usually fabricated by salt leaching or gas forming technique. However, it is not easy for cells to penetrate the scaffolds due to the poor interconnectivity of pores. To overcome these current limitations we utilized a rapid prototyping (RP) technique for fabricating tissue engineered scaffolds to treat critical defects. The RP technique resulted in the uniform distribution and systematic connection of pores, which enabled cells to penetrate the scaffold. Two kinds of materials were used. They were poly(ε-caprolactone) (PCL) and poly(d, l-lactic-glycolic acid) (PLGA), where PCL is known to have longer degradation time than PLGA. In vitro tests supported the biocompatibility of the scaffolds. A 12-week animal study involving various examinations of rabbit tibias such as micro-CT and staining showed that both PCL and PLGA resulted in successful bone regeneration. As expected, PLGA degraded faster than PCL, and consequently the tissues generated in the PLGA group were less dense than those in the PCL group. We concluded that slower degradation is preferable in bone tissue engineering, especially when treating critical defects, as mechanical support is needed until full regeneration has occurred.





Similar content being viewed by others
References
Persidis A. Tissue engineering. Nat Biotechnol. 1999;17:508–10.
Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cells Mater. 2009;5:29–40.
Chen G, Ushida T, Tateishi T. Scaffold design for tissue engineering. Macromol Biosci. 2002;2:67–77.
Pathiraja AG, Raju A. Biodegradable synthetic polymers for tissue engineering. Eur Cells Mater. 2003;5:1–16.
Sabir MI, Xu X, Li L. A review on biodegradable polymeric materials for bone tissue engineering applications. J Mater Sci. 2009;44:5713–24.
Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32:762–98.
Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43.
Barbanti SH, Carvalho Zavaglia CA, de Rezende Duek EA. Effect of salt leaching on PCL and PLGA(50/50) resorbable scaffolds. Mater Res. 2008;11:75–80.
Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(d,l lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–22.
Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42:396–402.
Yun H, Kim S, Hyun Y, Heo S, Shin J. Three-dimensional mesoporous-giantporous inorganic/organic composite scaffolds for tissue engineering. Chem Mater. 2007;19:6363–6.
Heo S, Kim S, Wei J, Kim DH, Hyun Y, Yun H, Kim HK, Yoon TR, Kim S, Park S, Shin JW, Shin J. In vitro and animal study of novel nano-hydroxyapatite/poly(ε-caprolactone) composite scaffolds fabricated by layer manufacturing process. Tissue Eng Part A. 2009;15:977–89.
De Santis R, Gloria A, Russo T, D’Amora U, Zeppetelli S, Dionigi C, Sytcheva A, Herrmannsdörfer T, Dediu V, Ambrosio L. A basic approach toward the development of nanocomposite magnetic scaffolds for advanced bone tissue engineering. J Appl Polym Sci. 2010;122:3599–605.
Kim GH, Son JG. 3D polycarprolactone (PCL) scaffold with hierarchical structure fabricated by a piezoelectric transducer (PZT)-assisted bioplotter. Appl Phys A. 2009;94:781–5.
Seyednejad H, Gawlitta D, Kuiper RV, Bruin A, Nostrum CF, Vermonden T, Dhert W, Hennink WE. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone). Biomaterials. 2012;33:4309–18.
Jia YT, Zhu XY, Liu QQ. In vitro degradation of electrospun fiber membranes of PCL/PVP blends. AMR. 2011;332–334:1330–4.
Sun H, Mei L, Song C, Cui X, Wang P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27:1735–40.
Kim J, McBride S, Tellis B, Alvarez-Urena P, Song YH, Dean DD, Sylvia VL, Elgendy H, Ong J, Hollinger JO. Rapid-prototyped PLGA/β-TCP/hydroxyapatite nanocomposite scaffolds in a rabbit femoral defect model. Biofabrication. 2012;4:1–11.
Baker SC, Rohman G, Southgate J, Cameron NR. The relationship between the mechanical properties and cell behaviour on PLGA and PCL scaffolds for bladder tissue engineering. Biomaterials. 2009;30:1321–8.
Hulbert SF, Morrison SJ, Klawitter JJ. Tissue reaction to three ceramics of porous and non-porous structures. J Biomed Mater Res. 1972;6:347–74.
Flatley TJ, Lynch KL, Benson M. Tissue response to implants of calcium phosphate ceramics in rabbit spine. Clin Orthop. 1983;179:246–52.
Slagada AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of they are and future trends. Macromol Biosci. 2004;4:743–65.
Yao J, Tao SL, Young MJ. Synthetic polymer scaffolds for stem cell transplantation in retinal tissue engineering. Polymers. 2011;3:899–914.
Hoffmeister BK, Smith SR, Handley SM, Rho JY. Anisotropy of Young’s modulus of human tibial cortical bone. Med Biol Eng Comput. 2000;38:333–8.
Rho JY, Ashman RB, Turner CH. Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J Biomech. 1993;26:111–9.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91.
Schieker M, Seitz H, Drosse I, Seitz S, Mutschler W. Biomaterials as scaffold for bone tissue engineering. Eur J Trauma. 2006;2:114–24.
Acknowledgments
This work was supported by the grants of Technology Innovation Program (10038667, Ministry of Knowledge Economy, ROK) and Priority Research Centers Program (2010-0020224, the Ministry of Education, Science and Technology).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Park, S.H., Park, D.S., Shin, J.W. et al. Scaffolds for bone tissue engineering fabricated from two different materials by the rapid prototyping technique: PCL versus PLGA. J Mater Sci: Mater Med 23, 2671–2678 (2012). https://doi.org/10.1007/s10856-012-4738-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4738-8