Abstract
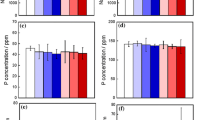
Solution-mediated reactions due to ionic substitutions are increasingly explored as a strategy to improve the biological performance of calcium phosphate-based materials. Yet, cellular response to well-defined dynamic changes of the ionic extracellular environment has so far not been carefully studied in a biomaterials context. In this work, we present kinetic data on how osteoblast-like SAOS-2 cellular activity and calcium-deficient hydroxyapatite (CDHA) influenced extracellular pH as well as extracellular concentrations of calcium and phosphate in standard in vitro conditions. Since cells were grown on membranes permeable to ions and proteins, they could share the same aqueous environment with CDHA, but still be physically separated from the material. In such culture conditions, it was observed that gradual material-induced adsorption of calcium and phosphate from the medium had only minor influence on cellular proliferation and alkaline phosphatase activity, but that competition for calcium and phosphate between cells and the biomaterial delayed and reduced significantly the cellular capacity to deposit calcium in the extracellular matrix. The presented work thus gives insights into how and to what extent solution-mediated reactions can influence cellular response, and this will be necessary to take into account when interpreting CDHA performance both in vitro and in vivo.






Similar content being viewed by others
References
Boanini E, Gazzano M, Bigi A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010;6:1882–94.
Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. PNAS. 2004;101:5140–5.
Aguirre A, Gonzalez A, Planell J, Engel E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem Biophys Res Co. 2010;393:156–61.
Beck GR Jr, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. PNAS. 2000;97:8352–7.
Fujita T, Izumo N, Fukuyama R, Meguro T, Nakamuta H, Kohno T, Koida M. Phosphate provides an extracellular signal that drives nuclear export of Runx2/Cbfa1 in bone cells. Biochem Biophys Res Co. 2001;280:348–52.
Liu YK, et al. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: implication for bone tissue engineering. Biomed Mater. 2009;4:1–8.
Allori AC, Sailon AM, Warren SM. Biological basis of bone formation, remodeling, and repair—part i: biochemical signaling molecules. Tissue Eng B. 2008;14:259–73.
Allori AC, Sailon AM, Warren SM. Biological basis of bone formation, remodeling, and repair—part II: extracellular matrix. Tissue Eng B. 2008;14:275–83.
Knabe C, et al. Evaluation of calcium phosphates and experimental calcium phosphate bone cements using osteogenic cultures. J Biomed Mater Res. 2000;52:498–508.
Hempel U, Reinstorf A, Poppe M, Fischer U, Gelinsky M, Pompe W, Wenzel KW. Proliferation and differentiation of osteoblasts on biocement D modified with collagen type I and citric acid. J Biomed Mat Res. 2004;71B:130–43.
Radin S, Reilly G, Bhargave G, Leboy PS, Ducheyne P. Osteogenic effects of bioactive glass on bone marrow stromal cells. J Biomed Mater Res. 2005;73A:21–9.
Engel E, del Valle S, Aparicio C, Altankov G, Asin L, Planell JA, Ginebra MP. Discerning the role of topography and ion exchange in cell response of bioactive tissue engineering scaffolds. Tissue Eng A. 2008;14:1341–51.
LeGeros RZ. Calcium-phosphate based osteoinductive biomaterials. Chem Rev. 2008;108:4742–53.
Yuan H, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. PNAS. 2010;107:13614–9.
Wong JY, Leach JB, Brown XQ. Balance of chemistry, topography, and mechanics at the cell–biomaterial interface: issues and challenges for assessing the role of substrate mechanics on cell response. Surf Sci. 2004;570:119–33.
El-Ghannam A, Ning CQ. Effect of bioactive ceramic dissolution on the mechanism of bone mineralization and guided tissue growth in vitro. J Biomed Mat Res. 2006;76A:386–97.
Habel B, Glaser R. Human osteoblast-like cells respond not only to the extracellular calcium concentration but also to its changing rate. Eur Biophys J. 1998;27:411–6.
Gustavsson J, Ginebra MP, Engel E, Planell J. Ion reactivity of calcium-deficient hydroxyapatite in standard cell culture media. Acta Biomat. 2011;7:4242–52.
Matsumot A, Tagushi H, Hisada Y. Effect of low-calcium environment on neonatal rat femora in culture. Toxicol in Vitro. 1991;5:51–62.
Farley JR, Hall SL, Tanner MA, Wegedal JE. Specific activity of skeletal alkaline phosphatase in human osteoblast-line cells regulated by phosphate, phosphate esters, and phosphate analogs and release of alkaline phosphatase activity inversely regulated by calcium. J Bone Miner Res. 1994;9:497–508.
Yoshimura Y, Hisada Y, Suzuki K, Deyama Y, Matsumoto A. Effect of a low-calcium environment on alkaline phosphatase activity in embryonic rat calvarial bone cells in culture. Arch Oral Biol. 1996;41:41–5.
Eklou-Kalonji E, Denis I, Lieberherr M, Pointillart A. Effects of extracellular calcium on the proliferation and differentiation of porcine osteoblasts in vitro. Cell Tissue Res. 1998;292:163–71.
Balint E, Szabo P, Marshall F, Sprague S. Glucose-induced inhibition of in vitro bone mineralization. Bone. 2001;28:21–8.
Gustavsson J, Planell J, Engel E. Ion-selective electrodes to monitor osteoblast-like cellular influence on the extracellular concentration of calcium. J Tissue Eng Regen Med. doi:10.1002/term550.
Español M, Perez RA, Montufar EB, Marichal C, Sacco A, Ginebra MP. Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater. 2009;5:2752–62.
Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarinoma cells. Hum Reprod. 2007;22:1304–9.
Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106 01 BSP). J Biol Chem. 1995;270:9420–8.
Chen PS, Toribara TY, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–8.
Khouja HI, Bevington A, Kemp GJ, Russell RGG. Calcium and orthophosphate deposits in vitro do not imply osteoblast mediated mineralization: mineralization by beta-glycerophosphate in the absence of osteoblasts. Bone. 1990;11:385–91.
Rodan SB, et al. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987;47:4961–6.
Fassina L, Visai L, Asti L, Benazzo F, Speziale P, Tanzi MC, Magenes G. Calcified matrix production by SAOS-2 cells inside a polyurethane porous scaffold, using a perfusion bioreactor. Tissue Eng. 2005;11:685–99.
Hausser HJ, Brenner RE. Phenotypic instability of Saos-2 cells in long-term culture. Biochem Biophys Res Co. 2005;333:216–22.
Schroder HC, Boreiko O, Krasko A, Reiber A, Schwertner H, Müller WEG. Mineralization of SaOS-2 cells on enzymatically (Silicatein) modified bioactive osteoblast-stimulating surfaces. J Biomed Mat Res. 2005;75B:387–92.
Orimo H, Shimada T. The role of tissue-nonspecific alkaline phosphatase in the phosphate-induced activation of alkaline phosphatase and mineralization in SaOS-2 human osteoblast-like cells. Mol Cell Biochem. 2008;315:51–60.
Thouverey C, Strzelecka-Kiliszek A, Balcerzak M, Buchet R, Pikula S. Matrix vesicle originate from apical membrane microvilli of mineralizing osteoblast-like Saos-2 cells. J Cell Biochem. 2009;106:127–38.
Chung C, Golub E, Forbes E, Tokuoka T, Shapiro I. Mechanism of action of β-glycerophosphate on bone cell mineralization. Calcified Tissue Int. 1992;51:305–11.
Arnett TR, Henderson B. Methods in bone biology. London: Chapman & Hall; 1998. p. 15–8.
Gentleman E, et al. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat Mater. 2009;8:763–70.
Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Gene Dev. 2005;19:1093–104.
Acknowledgments
Funding was obtained from the European Commission for projects NMP3-CT-2005-013912 and NMP-LA-2008-214402. J. G. acknowledges FI grant from the Generalitat de Catalunya. Support for the research by M. P. G. was received through the prize “ICREA Academia” for excellence in research, funded by the Generalitat de Catalunya.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gustavsson, J., Ginebra, M.P., Planell, J. et al. Osteoblast-like cellular response to dynamic changes in the ionic extracellular environment produced by calcium-deficient hydroxyapatite. J Mater Sci: Mater Med 23, 2509–2520 (2012). https://doi.org/10.1007/s10856-012-4705-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4705-4