Abstract
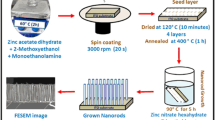
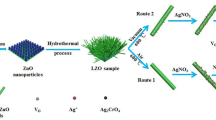
Orthorhombic Ag8SnS6/ZnO nanorods grown onto indium-tin-oxide conductive glass substrates were prepared using a simple chemical synthesis method for the application in photoelectrochemical salt-water splitting. For the deposition of ZnO nanorods on substrates, the concentrations of ammonium hydroxide in the solution bath were changed to obtain the pristine ZnO nanorods with good morphology for the growth of silver-tin-sulfide layer. Pristine ZnO nanorods prepared with concentration of 5.5 M for ammonium hydroxide in the reaction solution has the highest intensity of (0 0 2) crystal plane, which indicates that pristine ZnO nanorods sample has good morphology for the preparation of the Ag8SnS6 core/shell photoanode. From the results of X-ray diffraction patterns and energy-dispersive X-ray spectroscopy analysis for samples, with decreasing [Ag]/[Ag + Sn] molar ratio in the reaction solution, crystal phases of samples changed from Ag2S/SnS/ZnO, orthorhombic Ag8SnS6/ZnO to Ag8SnS6/SnS/ZnO mixing phases. The values of sample’s energy band gap were located in the range of 1.36 ~ 1.79 eV. At the [Ag]/[Ag + Sn] molar ratio of 0.4 in the reaction solution, the maximum photoelectrochemical activity of 0.79 mA cm−2 for samples was obtained with the external bias set at + 1.5 V vs. an Ag/AgCl electrode in 0.5 M NaCl aqueous solution. The photoelectrochemical activity of sample could remain at least 2000 s without any decay in the salt-water solution.










Similar content being viewed by others
References
A. Fujishima, K. Honda, Nature 238, 37 (1972)
A. Kudo, Catal. Surv. Asia 7, 31 (2003)
K.W. Cheng, C.H. Yeh, Int. J. Hydrog. Energy 37, 13638 (2012)
M. Grätzel, Nature 414, 338 (2001)
J. Li, N. Wu, Catal. Sci. Technol. 5, 1360 (2015)
S. Ichikawa, Int. J. Hydrog. Energy 22, 675 (1997)
K.W. Cheng, Y.H. Wu, T.H. Chiu, J. Power Sources 307, 329 (2016)
K.W. Cheng, S.W. Hong, ACS Appl. Mater. & Interfaces 10, 22130 (2018)
T. Ipeksac, F. Kaya, C. Kaya, Mater. Lett. 100, 11 (2013)
M. Kokotov, G. Hodes, J. Mater. Chem. 19, 3847 (2009)
Y. Zhang, X. Zhong, D. Zhang, W. Duan, X. Li, S. Zheng, J. Wang, Sol. Energy 166, 371 (2018)
Y. Hu, X. Yan, Y. Gu, X. Chen, Z. Bai, Z. Kang, F. Long, Y. Zhang, Appl. Surf. Sci. 339, 122 (2015)
D. Kumar, R. Bai, S. Chaudhary, D.K. Pandya, Mater. Today Energy 6, 105 (2017)
Y.C. Chen, K.H. Yang, C.Y. Huang, Z.J. Wu, Y.K. Hsu, Chem. Eng. J. 368, 746 (2019)
F.N.I. Sari, C. Lin, J.M. Ting, Chem. Eng. J. 368, 784 (2019)
M.E. Purbarani, F.N.I. Sari, J.M. Ting, Surf. Coat. Technol. 378, 125073 (2019)
C.R. Ke, J.S. Guo, Y.H. Su, J.M. Ting, Nanotechnology 27, 435405 (2016)
X. Yang, A. Wolcott, G. Wang, A. Sobo, R.C. Fitzmorris, F. Qian, J.Z. Zhang, Y. Li, Nano Lett. 9, 233 (2009)
Q. Ma, X. Peng, M. Zhu, X. Wang, Y. Wang, Y. Gao, H. Wang, J. Phys. D 52, 125503 (2018)
A. Brayek, S. Chaguetmi, M. Ghoul, I.B. Assaker, A. Souissi, L. Mouton, P. Beaunier, S. Nowak, F. Mammeri, R. Chtourou, S. Ammar, RSC. Adv. 6, 30919 (2016)
Y. Lai, L. Chi, Z. Li, Int. J. Hydrog. Energy 43, 22046 (2018)
Y.C. Liang, C.C. Chung, Y.J. Lo, C.C. Wang, Materials 9, 1014 (2016)
L.Y. Yeh, K.W. Cheng, J. Power Sources 275, 750 (2015)
K.W. Cheng, W.T. Tsai, Y.H. Wu, J. Power Sources 317, 81 (2016)
K.W. Cheng, J. Taiwan Inst. Chem. Eng. 87, 182 (2018)
D.W. Chen, K.Y. Lee, M.H. Tsai, T.Y. Lin, C.H. Chen, K.W. Cheng, Nanomaterials 9, 713 (2019)
Y. Tak, K. Yong, J. Phys. Chem. B 109, 19263 (2005)
A.F. Holleman, E. Wiberg, Inorganic Chemistry (Academic Press, San Diego, 2001).
D.R. Lide, H.P.R. Erederikse, CRC Handbook of Chemistry and Physics, 75th edn. (Boca Raton, CRC Press Inc, 1994).
W. Feng, L. Lin, H. Li, B. Chi, J. Pu, J. Li, Int. J. Hydrog. Energy 42, 3938 (2017)
V. Gurylev, C.Y. Su, T.P. Perng, Appl. Surf. Sci. 411, 279 (2017)
H. Feng, J. Wang, W. Fan, C. Zhang, Mater. Lett. 126, 67 (2014)
B. Thangaraju, P. Kaliannan, J. Phys. D 33, 1054 (2000)
B. Klahr, S. Gimenez, F. Fabregt-Santiago, T. Hamann, J. Bisquert, J. Am. Chem. Soc. 134, 4294 (2012)
P. Dias, L. Andrade, A. Mendes, Nano Energy 38, 218 (2017)
J. Kamimura, P. Bogdanoff, F.F. Abdi, J. Lähnemann, R. van de Krol, H. Riechert, L. Geelhaar, J. Phys. Chem. C 121, 12540 (2017)
A. Lasia, Electrochemical Impedance Spectroscopy and Its Applications (Springer, Heidelberg, 2014).
F. Tezcan, A. Mahmood, G. Kardas, J. Mater. Sci.: Mater. Electron. 29, 9547 (2018)
S. Sadhasivam, N. Anbarasan, M. Mukilin, P. Manivel, K. Jeganathan, Int. J. Hydrog. Energy 45, 30080 (2020)
B.J. Rani, A. Anusyia, M. Praveenkumar, S. Ravichandran, R.K. Guduru, G. Ravi, R. Yuvakkumar, J. Mater. Sci.: Mater. Electron. 30, 731 (2019)
B. Yadian, Y. Rao, B. Zhu, Z. Liu, Q. Liu, C.L. Gan, X. Chen, Y. Huang, Mater. Res. Bull. 96, 503 (2017)
Y. Zhang, T. Feng, X. Zhao, Q. Pei, T. Hao, W. Zhang, S. Wu, H. Mao, X.M. Song, J. Alloys Compd. 685, 581 (2016)
W. Hu, N.D. Quang, S. Majumder, E. Park, D. Kim, H.S. Choi, H.S. Chang, J. Alloys Comp. 819, 153349 (2020)
Acknowledgements
This work was sponsored by the Ministry of Science and Technology of Taiwan, under Grants of 106-2628-E-182-002-MY3(NERPD2G0293), 109-2221-E-182-001 (NERPD2K0021) and Chang Gung Memorial Hospital under Grant No. BMRP948.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, KY., Cheng, KW. Chemical synthesis of orthorhombic Ag8SnS6/zinc oxide nanorods photoanodes for photoelectrochemical salt-water splitting. J Mater Sci: Mater Electron 32, 10532–10548 (2021). https://doi.org/10.1007/s10854-021-05709-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05709-9