Abstract
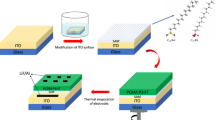
Self-assembled monolayers (SAMs) of benzoic acid based molecules are used to modify the metal oxide–polymer interface in a hybrid poly-3-hexylthiophene (P3HT)/TiO2 photovoltaic device structure. The effect of SAMs on current density is in accordance with expectation from the driving force for charge separation of metal oxide–polymer interface in a hybrid poly-3-hexylthiophene (P3HT)/TiO2 photovoltaic device. However, the effect of monolayers on open circuit voltage is quite unexpected from the interfacial energetics as all the monolayers improve the open circuit voltage in spite of different sign of the interfacial dipole for different SAMs. This suggests that the monolayers have additional functions. Overall device performance is enhanced by more than a factor of two using a SAM with permanent dipole pointing towards the TiO2 surface or pointing towards polymer when compared to a control device with no interface modifiers. This study concludes that the SAM layer has two functions that are to shift the position of the conduction band of the porous TiO2 relative to the polymer HOMO level so as to influence interfacial charge separation and to act as a barrier layer, insulating back electron transfer from the TiO2 to the polymer. Both effects can benefit the performance of hybrid polymer metal oxide solar cells.





Similar content being viewed by others
References
S.H. Liao, H.J. Jhuo, P.N. Yeh, Y.S. Cheng, Y.L. Li, Y.H. Lee, S. Sharma, S.A. Chen, Sci. Rep. 4(6813), 1–7 (2014)
T. Xu, Q. Qiao, Energy Environ. Sci. 4(8), 2700–2720 (2011)
C.C. Chueh, C.Z. Li, A.K.Y. Jen, Energy Environ. Sci. 8(4), 1160–1189 (2015)
S. Chen, J.R. Manders, S.W. Tsang, F. So, J. Mater. Chem. 22(46), 24202–24212 (2012)
P. Ravirajan, A.M. Peiró, M.K. Nazeeruddin, M. Graetzel, D.D.C. Bradley, J.R. Durrant, J. Nelson, J. Phys. Chem. B 110(15), 7635–7639 (2006)
J. Krüger, U. Bach, M. Grätzel, Adv. Mater. 12(6), 447–451 (2000)
C. Goh, S.R. Scully, M.D. McGehee, J. Appl. Phys. 101(11), 114503 (2007)
S. Loheeswaran, K. Balashangar, J. Jevirshan, P. Ravirajan, J. Nanoelectron. Optoelectron. 8(6), 484–488 (2013)
M. Thanihaichelvan, K. Sockiah, K. Balashangar, P. Ravirajan, J. Mater. Sci.: Mater. Electron. 26(6), 3558–3563 (2015)
S. Khodabakhsh, B. Sanderson, J. Nelson, T.S. Jones, Adv. Funct. Mater. 16(1), 95–100 (2006)
J.S. Kim, J.H. Park, J.H. Lee, J. Jo, D.Y. Kim, K. Cho, Appl. Phys. Lett. 91, 112111 (2007)
B. Delgertsetseg, N. Javkhlantugs, E. Enkhtur, Y. Yokokura, T. Ooba, K. Ueda, C. Ganzorig, M. Sakomura, Org. Electron. 23, 164–170 (2015)
A. Khassanov, H.G. Steinrück, T. Schmaltz, A. Magerl, M. Halik, Acc. Chem. Res. 48(7), 1901–1908 (2015)
S. Khodabakhsh, D. Poplavskyy, S. Heutz, J. Nelson, D.D.C. Bradley, H. Murata, Adv. Funct. Mater. 14(12), 1205–1210 (2004)
D. Cahen, A. Kahn, Adv. Mater. 15(4), 271–277 (2003)
A. Abrusci, S.D. Stranks, P. Docampo, H.L. Yip, A.K.Y. Jen, H.J. Snaith, Nano Lett. 13(7), 3124–3128 (2013)
S.K. Hau, Y.J. Cheng, H.L. Yip, Y. Zhang, H. Ma, A.K.Y. Jen, ACS Appl. Mater. Interfaces 2(7), 1892–1902 (2010)
M. Carrara, F. Nüesch, L. Zuppiroli, Synth. Met. 121(1–3), 1633–1634 (2001)
G.R.R.A. Kumara, K. Tennakone, V.P.S. Perera, A. Konno, S. Kaneko, M. Okuya, J. Phys. D Appl. Phys. 34(6), 868–873 (2001)
J.N. Clifford, E. Palomares, M.K. Nazeeruddin, M. Gratzel, J. Nelson, X. Li, N.J. Long, J.R. Durrant, J. Am. Chem. Soc. 126(16), 5225–5233 (2004)
E. Palomares, C.N. Clifford, A. Haque, T. Lutz, J.R. Durrant, Chem. Commun. 14, 1464–1465 (2002)
C. Goh, S.R. Scully, M.D. McGehee, J. Appl. Phys. Lett. 101, 114503–114515 (2007)
T. Ishwara, D.D.C. Bradley, J. Nelson, P. Ravirajan, I. Vanseveren, T. Cleij, D. Vanderzande, L. Lutsen, S. Tierney, M. Heeney, I. McCulloch, Appl. Phys. Lett. 92(5), 053308 (2008)
M.D. McGehee, MRS Bull. 34(2), 95–100 (2009)
Y. Kim, A.M. Ballantyne, J. Nelson, D.D.C. Bradley, Org. Electron. 10(1), 205–209 (2009)
M. Shalom, S. Rühle, I. Hod, S. Yahav, A. Zaban, J. Am. Chem. Soc. 131(29), 9876–9877 (2009)
P. Wang, S.M. Zakeeruddin, J.E. Moser, M.K. Nazeeruddin, T. Sekiguchi, M. Gratzel, Nat. Mater. 2, 402–407 (2003)
A.M. Peiro, P. Ravirajan, K. Govender, D.S. Boyle, P. O’Brien, D.D.C. Bradley, J. Nelson, J.R. Durrant, J. Mater. Chem. 16(21), 2088–2096 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loheeswaran, S., Thanihaichelvan, M., Ravirajan, P. et al. Controlling recombination kinetics of hybrid poly-3-hexylthiophene (P3HT)/titanium dioxide solar cells by self-assembled monolayers. J Mater Sci: Mater Electron 28, 4732–4737 (2017). https://doi.org/10.1007/s10854-016-6116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6116-7