Abstract
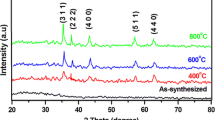
The main objective of this study deals with the synthesis and characterization of Ni1−xCoxFe2O4 (x = 0.3, 0.5, 0.7) by a facile novel co-precipitation method using citrate as chelating agent. TG/DT analysis was performed for the as-prepared sample, which shows the calcination is necessary for the formation of pure nickel cobalt ferrite nanoparticles. The synthesized powders were calcinated at 400, 600 and 800 °C for 3 h in air and were characterized by X-ray diffraction (XRD) which confirmed the formation of cubic spinel structure of ferrites. From XRD it confirms that Ni1−xCoxFe2O4 nano particles belong to spinel type lattice of space group Fd3m. The linear relationship between particle size and calcination temperatures of Ni1−xCoxFe2O4 nanoparticles was observed. The Ni1−xCoxFe2O4 nano particles calcinated at 600 °C were further characterized by using techniques field emission scanning electron microscope (FESEM) with EDAX, field emission transmission electron microscope (FETEM) with SAED pattern, dynamic light scattering zeta potential, X-ray photoelectron spectroscopy and cyclic voltammetry (CV). The surface morphology of Ni1−xCoxFe2O4 studied through FESEM and FETEM indicate that the particles are found crystalline and are in cubic shape. EDAX analysis revealed the presence of Ni, Co, Fe and O. Zeta potential exposes the good stability of the prepared Ni1−xCoxFe2O4 nanoparticles. Capacitance value 865 Fg−1 was observed for the scanning rate of 2 mV s−1 from the CV study and concluded it can be used for super capacitor application.










Similar content being viewed by others
References
H. Wang, F. Zhang, W. Zhang, X. Wang, Z. Lu, Z. Qian, Y. Sui, D. Dong, W. Su, J. Cryst. Growth (2006). doi:10.1016/j.jcrysgro.2006.05.002
K. Nejati, R. Zabihi, Chem. Cent. J. (2012). doi:10.1186/1752-153X-6-23
S. Mishra, N. Karak, T.K. Kundu, D. Das, N. Maity, D. Chkravorty, Mater. Lett. 60, 1111 (2006)
T.R. Mandlimath, B. Gopal, J. Mol. Catal. A Chem. 350, 9–15 (2011)
P. Derakhshi, S.A. Khorrami, R. Lotfi, Appl. Sci. J. 16(2), 156–159 (2012)
K. Krieble, T. Schaeffer, J. Appl. Phys. 97, 10F101 (2005)
L. Zhao, Y. Cui, H. Yang, L. Yu, W. Jin, S. Feng, Mater. Lett. 60, 104 (2006)
A. Thakur, P. Mathur, M. Singh, J. Phys. Chem. Solids 68, 378 (2007)
G. Kumar, J. Chand, S. Verma, M. Singh, J. Phys. D Appl. Phys. 42, 155001 (2009)
S.K. Pradhan, S. Bid, M. Gateshki, V. Petkov, Mater. Chem. Phys. 93, 224 (2005)
D.S. Jung, Y.C. Kang, J. Magn. Magn. Mater. 321, 619 (2009)
L. Chen, H. Dai, Y. Shen, J. Bai, J. Alloys Compd. 491, 33 (2010)
Y. Koseoğlu, A. Baykal, F. Gozuak, H. Kavas, Polyhedron 28, 2887 (2009)
M.H. Sousa, E. Hasmonay, J. Depeyrot, F. Tourinho, J.C. Bacri, E. Dubois, R. Perzynski, Y.L. Raikher, J. Magn. Magn. Mater. 242–245, 572 (2002)
C. Ramankutty, S. Sugunan, Appl. Catal. A Gen. 218, 39 (2001)
A. Gaffoor, D. Ravinder, Int. J. Eng. Res. Appl. 4(4), 73–79 (2014)
N.B. Velhal, N.D. Patil, A.R. Shelke, N.G. Deshpande, V.R. Puri, AIP Adv (2015). doi:10.1063/1.4931908
B.D. Cullity, Elements of X-ray diffraction (Addison-Wesley, Reading, 1978)
M.A. Salem, M.S. Salim, R.M.E. Okr, M. Ashoush, H.M. Talaat, M.M. El-Okr, J. Magn. Magn. Mater. 323, 920–926 (2011)
S.S. Umare, R.S. Ningthoujam, S.J. Sharma, S. Shrivastava, S. Kurian, N.S. Gajbhiye, Hyperfine Interact. (2008). doi:10.1007/s10751-008-9796-4
H.P. Klug, L.E. Alexander, X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd edn. (Wiley, New York, 1974)
A.C.F. Costa, E. Tortella, M.R. Morelli, E.F. Neto, R.H.G.A. Kiminami, Mater. Res. 7, 523 (2004)
S. Singhal, J. Singh, S.K. Barthwal, K. Chandra, J. Solid State Chem. 178, 3183–3189 (2005)
P.P. Hankare, K.R. Sanadi, K.M. Garadkar, D.R. Patil, I.S. Mulla, J. Alloys Compd. 553, 383–388 (2013)
M.A. Ati, H. Khudhair, S. Dabagh, R.M. Rosnan, A.A. Ati, Int. J. Sci. Eng. Res. 5(9), 927–930 (2014)
K. Maaz, W. Khalid, A. Mumtaz, S.K. Hasanain, J. Liu, J.L. Duan, Phys. E 41, 593–599 (2009)
C. Singh, A. Goyal, S. Singhal, RSC. Nanoscale (2014). doi:10.1039/c4nr01730g
P. Sivagurunathan, S.R. Gibin, J. Mater. Sci. Mater. Electron. (2015). doi:10.1007/s10854-015-4065-1
P. Sivagurunathan, S.R. Gibin, J. Mater. Sci. Mater. Electron. (2016). doi:10.1007/s10854-016-4915-5
K. Sathishkumar, N. Shanmugam, N. Kannadasan, S. Cholan, G. Viruthagiri, J. Mater. Sci. Mater. Electron. (2015). doi:10.1007/s10854-014-2624-5
S.C. Pang, B.H. Wee, S.F. Chin, Int. J. Electro. Chem. (2011). doi:10.4061/2011/397685
S.-K. Chang, Z. Zainal, K.B. Tan, N.A. Yousaf, W.M. Daud, W. Yousaff, S.R.S. Prabaharan, Int. J. Energy Res. (2012). doi:10.1002/er.3339
Seema Joshi, Manoj Kumar, J. Supercond. Novel Mag. 29(6), 1561–1572 (2016)
J.S. Corneille, J.-W. He, D.W. Goodman, Surf. Sci. 338, 211–224 (1995)
Seema Joshi, V.B. Kamble, M. Kumar, A.M. Umarji, G. Srivastava, J. Alloys Compd. (2015). doi:10.1016/j.jallcom.2015.09.119
F. Tudorachea, P.D. Popab, M. Dobromira, F. Iacomia, Mater. Sci. Eng. B 178, 1334–1338 (2013)
S. Joshi, M. Kumar, J. Supercond. Nov. Magn. (2015). doi:10.1007/s10948-016-442-1
B. Mojic, K.P. Giannakopoulosb, Z. Cvejic, V.V. Srdic, Ceram. Int. 38, 6635–6641 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gibin, S.R., Sivagurunathan, P. Synthesis and characterization of nickel cobalt ferrite (Ni1−xCoxFe2O4) nano particles by co-precipitation method with citrate as chelating agent. J Mater Sci: Mater Electron 28, 1985–1996 (2017). https://doi.org/10.1007/s10854-016-5755-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5755-z