Abstract
Pure zinc oxide, single-doped zinc oxide as 2 wt% of Lanthanum as (La DZ NPs), 2 wt% of Samarium (Sm DZ NPs), and 2 wt% of Strontium as (Sr DZ NPs), and tri-doped (Sm, La, Sr, T DZ NPs) were synthesized with a hydrothermal method. Additionally, these nanomaterials are used as an effective photocatalytic for the degradation of Reactive Red 43. These nanomaterials’ optical, particle size distribution, structural properties, and morphology were analyzed using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), ultraviolet (UV) light, photoluminescence (PL), scanning electron microscopy (SEM) and energy-dispersive X-ray (XPS), transmission electron microscopy (TEM), and the point of zero charges (pHpzc). Molecular modeling simulation was calculated using density functional theory (DFT) to confirm some characterization. Moreover, these studies showed the crystal structure parameters changed with doped nanomaterials, and the experimental band gap fit theoretical calculation and demonstrated the reason for the widening of the band gap. An enhancement in the surface area of Sr DZ NPs recorded high value (SBET = 37.43 m2/g) indicated that it can be used as an efficient photocatalyst, where Sr DZ NPs showed the best photodegradation % of Reactive Red 43 dye with 93.43% compared to PZ (72.88%), La DZ NPs (52.54 3%), Sm DZ NPs (31.99%), and La, Sm, Sr T DZ NPs (20.55%). Furthermore, the pseudo-first-order kinetic model better fits the R2 values. Finally, the mechanism of degradation has been related to electronic configuration. In addition, the recyclability showed stability of nanomaterials under UV irradiation.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid development of industrialization has triggered multiple ecological risks. As a result, it was found that water contamination is at the root of most of the global environmental problems. Due to the extremely large amount of dye effluent released by textile dyeing industries, which produce 700,000 tons of synthetic dyes annually, large volumes of wastewater are discharged into the environment [1]. In general, dyes are hazardous because of their strong resistance to light, temperature, chemicals, and microbial attack [2]. Dye degradation from wastewater can help resolve many environmental issues related to human and aquatic life [3]. Many strategies have been used to remove color pollutants from water, such as chemicals, photochemicals, biological, and physicals to treat the contaminated areas [4, 5]. Among the removal strategies, photodegradation is one of the most effective ways to eliminate pollutants from wastewater [6, 7]. Combining adsorption with photocatalytic degradation is one of the most economical approaches to decontaminating wastewater [8]. In recent years, many materials have been developed in various shapes and structures [9]. Additionally, several semiconductors have been used in the photocatalytic degradation of dyes contaminations, including ZnO, TiO2, CdS, SnO2, CuO, NiO FeO3 etc.[10,11,12]. Among these metal oxides, it was reported that the SnO2 was produced by changing the solvent in the precursor solution using the precipitation method. The comparison investigation found that as the solvent was changed from 23 to 6 nm, the average crystallite size changed, with a tetragonal crystal structure. The photodegradation rate of MB dye revealed that SnO2 nanostructures made using IPA rather than DI and ethanol had the highest photodegradation efficiency [13]. The authors used a simple co-precipitation approach to synthesize Ce-doped ZnO nanostructures with varying doping amounts. Under visible light irradiation, the Zn0.94Ce0.06O sample photocatalyzed Methylene blue (MB) dye was 9.1 times faster than the undoped ZnO. An increase in PL intensity causes an increase in photocatalytic activity. As a result, Ce-doping increases the degradation rate, making ZnO a viable photocatalyst for removing organic contaminants in water [14]. ZnO has many features to become an environmentally friendly metal oxide and properties, such as inexpensive, safe, low toxicity, biodegradable, and easily prepared [15]. Moreover, Zinc oxide (ZnO) has emerged as an effective photocatalyst due to its higher H2O2 generation compared to other metal oxide nanomaterials [16, 17]. ZnO modified by adding dopants metals, such as Ce, Cu, Ag, Mn, Ni, Fe, Sr, and Lanthanides to enhance their photocatalytic activity [18]. The ZnO nanomaterials were synthesized using various strategies, including co-precipitation, combustion, hydrothermal, sol–gel, polyol, and sonochemical [19]. Hydrothermal synthesis is one of the most common methods for obtaining nanomaterials based on solution reactions, and nanomaterials can be produced at a range of temperatures and pressure from low to high [20]. In addition, this method considers environmental safety. It is inexpensive due to its use of water as a solvent and gives us good control over the particle shape and size [21]. There are significant advantages of the hydrothermal synthesis method over others in that the compositions of nanomaterials to be synthesized can be well controlled through liquid phase or multiphase chemical reactions [22]. It was reported that hydrothermal fabrication methods are major routes for synthesizing unique ZnO shapes because it offers both the possibility of rigorous control over crystallite growth and relatively easy upscaling [23]. Four parameters affect crystallization: phase barrier, capping molecules, time, and temperature [24]. The hydrothermal method is also beneficial in a pressure vessel, also known as an autoclave, where varying temperatures and pressure control processing conditions [25]. Researchers have performed doping in the ZnO to obtain a wide range of properties and use for the material. Metal doping is a simple way to increase the efficiency of ZnO nanomaterials in various applications. Firstly, photocatalytic characteristics can be controlled by selecting alkaline earth metal elements as dopants. Indeed, using cations as doping metals with a greater ion radius than Zn+2 radius results in the formation of considerable lattice defects due to ionic radius mismatch between cations and Zn+2 and charge compensation, which may impact the photocatalytic capabilities of ZnO. Additionally, it was known that Sr+2 has a greater radius (2.45Å) than Zn+2 which can affect ZnO's optical characteristics [26]. Moreover, the second group involved the insertion of cations of rare-earth metal within the ZnO matrix that deserve special attention and analyses the light absorption and changes in structural characteristics, defects on the surface, crystallite sizes, and thus the photocatalytic properties; and create intra-4f optical transitions due to the half-filled f-orbital [27, 28]. Optical and photocatalytic properties are recognized to be directly related to one another. Thus, we anticipate seeing these results in the efficiency with which dye is degraded from doped ZnO nanostructures. Specifically, the ZnO doping will produce a larger band gap energy and less charge carrier recombination, improving photocatalytic efficiency [29]. Theoretical calculations based on density functional theory (DFT) have been widely used to establish the relationship between photo electrocatalysts' structure and catalytic activity. These computations used first principles to predict the electronic characteristics of doped ZnO [30].
In this study, we used a hydrothermal method for producing single-doped ZnO and tri-doped ZnO nanomaterials with different metals, such as Sr metal as alkaline earth metals and Sm and La metals as rare earth metals. The obtained composite was investigated to determine the structure, optical properties, electronic properties, morphology, and surface area, and compared these experimental results with the theoretical calculation. Finally, the experimental study of the photodegradation of reactive red 43 and the mechanisms of photodegradation were discussed. Moreover, the DFT calculation showed the difference in crystal structures, electronic properties, and optical properties of the nanomaterials.
Materials and methods
Materials
Zinc acetate dihydrate [Zn (CH3COO)2·2H2O], Lanthanum (III) nitrate hexahydrate [La (NO3)3·6H2O], Samarium (III) nitrate hexahydrate [Sm (NO3)3·6H2O], Strontium nitrate [Sr (NO3)], Sodium hydroxide [NaOH], Acetic acid [CH3COOH] (99.9%), Reactive Red43[C26H17ClN7Na3O11S3], and Distilled water (D.W) used all chemical with no extra purification.
Synthesis technique
Synthesis of pure ZnO nanomaterials (PZ NPs)
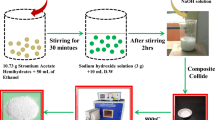
Pure ZnO was prepared by dissolving 5gm of Zinc acetate dihydrate in 250 mL of Distilled water under magnetic stirring for 30 min at 25 °C. Then, 2.5 mL acetic acid was added and further stirred for 10min. Then used, NaOH to adjust the pH range (10–11); at this, a white precipitate was formed, followed by 30min of stirring. The mixture was placed in a stainless-steel autoclave lined with Teflon and heated to 160 °C for 3 h. After cooling to room temperature, the yield was removed, filtered, and washed by D.W. many times. Finally, the product obtained was dried at 80 °C.
Synthesis of single doped ZnO as Sm doped ZnO (Sm DZ NPs), la doped ZnO (La DZ NPs), and Sr doped ZnO (Sr DZ NPs) nanomaterials
We generated single doped ZnO by dissolving 5 gm of Zinc acetate dihydrate in 250 ml of Distilled water at room temperature with magnetic stirring for 30 min. The mixture was agitated for 10 min after adding 2.5 ml of acetic acid. As a dopant, add 2.0 mol% Lanthanum (III) nitrate hexahydrate to the preceding mixture and stir until dissolved. Similarly, we completed the experiment as in the case of the synthesis of Pure ZnO, and the yield was labeled as (La DZ NPs). In addition, in the case of preparing Sm doped ZnO, add 2.0 mol% of samarium (III) nitrate hexahydrate labeled as (Sm DZ NPs), while in the case of Sr doped ZnO added 2.0 mol% of strontium nitrate labeled as (Sr DZ NPs).
Synthesis of tri-doped ZnO (la, Sm, Sr Tri- doped ZnO Nano particles (Sm, La, Sr TDZ NPs)
Tri-doped ZnO was prepared by dissolving 5gm of Zinc acetate dihydrate in 250mL of distilled water under magnetic stirring for 30min. at 25 °C. After that, 2.5 mL of acetic acid was added, and the mixture was further stirred for 10 min., then added 2.0 mol% of lanthanum (III) nitrate hexahydrate, 2.0 mol% of samarium (III) nitrate hexahydrate, 2.0 mol% of strontium nitrate (the molar ratio of La: Sm: Sr is 1:1:1) under vigorous stirring until dissolved. Then, NaOH was used to adjust the pH range (10–11) at this white precipitate, followed by stirring for 30min. A Teflon-lined stainless-steel autoclave received the mixture, which was closed for 24h at 200 °C. The yield was washed with D.W. and dried at 80 °C shown in.
Characterization
The synthesized of PZ, Sm DZ NPs, La DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs were affirmed by numerous characterization techniques. The structural properties were investigated by X-ray diffraction (XRD), (PANalytical X'Pert Pro); using Cu-Kα irradiation in a scanning range of 2θ between 10 and 80. The solid phase FT-IR spectrum was investigated by (Perkin Elmer-Spectrum). A Raman spectrum (InVia; Renishaw) was recorded with a laser beam having an excitation wavelength of 514 nm. Additionally, the optical properties were studied by using a spectrophotometer (JASCO V-770), the optical bandgaps were estimated from linear extrapolation of Tauc plots, and photoluminescence (PL) spectroscopy ([RF-5301 Pc) carried with irradiation source at excitation wavelength 345 nm. The binding state of doped metal was distinguished with the X-ray photoelectron spectroscopy (XPS) (HP 5950A, Hewlett-pakard firm). The surface morphology was determined by SEM-(Hitachi, S-4800) attached with Energy-dispersive X-ray (EDX) that using with X-ray photoelectron spectroscopy (XPS-Thermo Electron Corporation Theta Probe) to evaluate the compound elements in the synthesized photocatalysts. The zeta potential is represented by ZS Malvern, A zetasizer attached to an automatic titrator (Malvern MPT-2). By utilizing nitrogen as the adsorbate at 77.4 K in a Fisons SORPTOMATIC 1900 volumetric adsorption–desorption apparatus, the samples' specific surface areas and pore size distributions were calculated.
Theoretical study
The CASTEP module in the materials studio was used to compute this work. The generalized gradient approximation (GGA + U) with the Perdew-Burke-Ernzerh (PBE) function was used to explain the exchange–correlation energy. By employing a cutoff energy of 380 eV and a selection of the Brillouin zone with 5 × 5 × 4 k-points in the Bronkhorst-pack approach. We select the DFT/GGA + U technique. In this study, Ud,zn = 10 eV, Up,O = 6.5 eV, Uf,,o doped metal = 6 eV, which were fine for computation accuracy. Methods of computation of the hexagonal wurtzite structure with the P63mc space group symmetry system is the most stable Zinc oxide at room temperature and pressure. A pair of Zn atoms and a pair of Oxygen atoms make up the primitive cell. PZ, La DZ NPs, and Sr DZ NPs were all modeled in this work using a 2 × 2 × 2 supercell; atoms of La and Sr are exchanged for their neighboring Zn atoms.
Photocatalytic degradation procedure
Photocatalysts activities of PZ, Sm DZ NPs, La DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs investigated degradation of Reactive red 43. Using 20 ppm as the initial concentration in 50 mL of dye, adjust pH at 8, and the photocatalyst was added at a dose of 0.05 g/L. Reactive red 43dye and photocatalyst were magnetically stirred for 1h in the dark to establish absorption/desorption equilibrium. A model UV–visible spectrometer examined the absorption spectrum of an aliquot 3 mL of the deteriorated solution at intervals. The photodegradation of RR43 was studied by measuring the change in absorption intensity using UV–visible spectroscopy every 20 min for a period of 120 min. The experiment was carried out in the same way for both pure and doped ZnO nanomaterials.
Results and discussion
Characterization
X-ray diffraction (XRD)
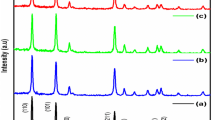
Figure 1a exhibited diffraction patterns of XRD data for PZ, Sm DZ NPs, La DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs. By analyzing these patterns, crystallographic properties were studied, such as average crystallite size, lattice parameters, and the nature of the sample. We found Card No. 01-079-0207; Zinc Oxide; Hexagonal corresponds well with diffraction peaks obtained for synthesized samples, the peaks of Sm, La, Sr TDZ NPs located at 31.72, 34.36, 36.20, 47.54, 56.53, 62.80, 66.33, 67.86, 72.60, 74.89and 76.91planes (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) and (202) can be attributed to the hexagonal wurtzite; this result indicated the doping does not change the crystalline phases of ZnO [31, 32]. No additional peak was detected in all samples, demonstrating the crystallinity and purity of the produced samples [33]. The three most intense peaks (100), (002), and (101) of the total samples are displayed in Fig. 1b as magnified XRD patterns. The single and tri-doped ZnO showed the high three peaks intensity reduced and shifted to the higher 2θ angle when compared with Pure ZnO [34], which indicated that these metal Sm3+, La3+, and Sr2+ ions are incorporated into crystals lattice of the ZnO [35]. The average crystallite size was calculated by using Scherer’s equation:
where D is the average crystal size in nm, λ is the Cu-Kα wavelength (0.15406 nm), β is the full width at half maximum, and θ is the direction angle. In Table 1, it was found that an increase in the average crystallite size was due to the addition of dopants Sm3+, La3+, and Sr2+ ions, a similar result reported in [29]. The lattice parameters and volume of single-doped and tri-doped unit cell exhibited values slightly smaller than undoped ZnO, as shown in Table 1. The slight difference in lattice parameters revealed that Sm3+, La3+, and Sr2+ ions are successfully incorporated into the ZnO lattice where it, interstitially, substitutes the Zn2+ ion sites as the ionic radius of Sm3+ (1.08 Å), La3+ (1.06 Å), and Sr2+ (2.45 Å) is larger than that of Zn2+ (0.74 Å). The shift in peaks and changes in lattice characteristics indicate that Sm3+, La3+, and Sr2+ ions are replacing Zn2+ ions [36].
FT-IR spectra
The FT-IR spectroscopy was analyzed to explore the effect of different metals, such as (Sm, La, and Sr) doping, on ZnO, shown in Fig. 2a. The vibrational properties of synthesis samples were measured between 4000 and 400 cm−1. Broad bands are noticed at around 3309.42, 3353.97, 3328.08, and 3422.87 cm−1 for PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs, respectively, and therefore, these bands are corresponding to the stretching vibration of the O–H in water [25]. Furthermore, bands were observed in the range 1502.06, 1395.40, and 700–1100 cm−1corresponding to the starting materials [37]. Additionally, Zn–O stretching absorption bands are represented at 464.63, 455.14, 512.285, 4 65.98, and 514.93 cm−1 for PZ, La DZ NPs, Sm DZ NPs, Sr DZ NP, and Sm, La, Sr TDZ NPs, respectively [38, 39]. Further, the doped nanomaterials exhibited a slight shift in the absorption band, where samarium, lanthanum, and strontium atoms are heavier than the Zn atom. These changes in the band's position can be explained by the changes in the bond length when a Sm, La, or Sr atom is replaced by a Zn atom. Finally, the shifting of these bands indicated that doping was formed.
Raman spectroscopy
According to group theory, the Raman scattering spectroscopy was used to investigate Nano-scale structures and vibrational properties of doping system. The optical phonon modes in Wurtzite type ZnO at the Brillouin T point are represented by T = A1 + 2B1 + E1 + 2E2, Where A1 and E1 are polar Raman active modes with transverse optical (TO) and longitudinal optical (LO) phonons. At the same time, B1 is the Raman inactive mode, and E 2(low) and E 2(high) are nonpolar. Raman detects crystal stress and defect development [37]. Figure 2b exhibited the Raman spectra for PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, and Sr TDZ NPs, respectively, with the position of phonon vibration frequencies. The strong peaks observed around 441 cm−1 are assigned to the E 2 (high) and showed peaks at 96.50, 98.50, 96.88, 96.25, and 98.84 cm−1 and assigned to the E 2(low). Additionally, peaks at 332.5, 333.75, and 331.25 cm−1 correspond to the multi-phonon scattering process E2(high)-E2(low) mode of vibration, which is a characteristic Raman active for the wurtzite hexagonal phase of ZnO. All the samples show these peaks are due to the Zn and O sub-lattice [34]. Moreover, peaks at 378.75, 383.25, 393.26, 385.5, and 375.76 cm−1 for PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs, respectively, indicating the A1(TO) phonon mode that is usually represented by the defect of the zinc interstitial and oxygen vacancy in the ZnO lattice. Similarly, there are peaks due to E1(LO) mode, which is associated with the impurities and formation of defects at 570.02, 597.57, and 573.25 cm−1 for PZ, La DZ NPs, Sm DZ NPs, and Sr DZ NPs, respectively [40]. In single and Tri-doped, a reduction of intensity and shift in some peaks occurred because of the incorporation of Sm3+, La3+, and Sr2+ into the ZnO lattice. These peaks indicated an increase in defect formation in the synthesized nanomaterials [39].
Ultraviolet–visible spectroscopy (UV–vis)
The UV–vis of PZ, Sm DZ NPs, La DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs were illustrated in Fig. 3a. As represented in Fig. 3a, an absorption band of PZ was observed at 380 nm, but in single-doped samples, as La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs exhibited absorption bands lower than PZ at 375.86, 364.17, 377.25, and 367.67 nm, respectively [41]. All doped ZnO displayed a blue shift toward a lower wavelength than pure ZnO. The band gap of doped ZnO has widened, as evidenced by the blue shift. We also used the Tauc relationship (Eq. 2) to determine the energy band gaps in pure ZnO and doped synthesized ZnO:
where α is the absorption coefficient, Eg is the optical band gap, and v is the frequency of a photon. We have taken a value of ½ for a direct band gap semiconductor. Tauc's plot of (αhv)2 vs. photon energy (hν) shows the Eg. The calculated Eg values are 2.86, 2.89, 2.93, 2.98, and 3.02 eV for PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and La, Sm, Sr TDZ NPs, respectively shown in Fig. 3b. The Eg of doped ZnO increased compared with pure ZnO as a result of the presence of the oxygen stoichiometry and the quantum confinement effect [29]. Because the value of the band gap rises, the probability that the electron–hole pairs will have greater redox potentials rises along with it, leading to an increase in photocatalytic activity [42].
Photoluminescence (PL)
Figure 4 depicts the fluctuation in the PL intensity of PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs. and La, Sm, Sr TDZ NPs nanomaterials with incident photon wavelength. In visible region peaks at 448 nm due to the recombination of the free exactions on the ZnO surface [43]. Additionally, the observed peak in the blue region at 469 nm is due to the transition of electrons. Dopant ions Sm3+, La3+, and Sr2+, induce perturbations in the ZnO crystal, and new energy levels are also created by introducing these ions [41]. Moreover, strong peaks related to the high density of oxygen vacancies during the preparation were observed in the green region at 470.56, 470.48, 469.54, 470.52, and 469.53 nm for La DZ NPs, Sm DZ NPs, Sr DZ NPs. and La, Sm, Sr TDZ NPs, respectively. By comparing doped ZnO and pure ZnO, it was found that the PL intensity increased when Sm3+, La3+, and Sr2+ ions were combined into the ZnO crystal structure, which may be due to the defects of oxygen vacancies. It is widely accepted that nanomaterials of ZnO contain defects, according to these results, the synthesized. Nanomaterials possess the capacity to promote photocatalysis.
XPS analysis
The valence and chemical composition of an element of a doping metal have been investigated using XPS measurement. All XPS spectra were calibrated for binding energy using the carbon peak at 284.87 eV as a reference. According to Fig. 5a, the undoped ZnO survey spectrum displays peaks assigned to the Zn 2p state, O 1s state, and C 1s state. The XPS spectrum of Zn 2p state for PZ displayed doublet peaks at 1026.20 eV corresponding to 2p3/2 states correlated with the hydroxyl group linkage of zinc within ZnO and 1048.82 eV for 2p1/2 states corresponding to the atomic linkage of zinc with oxygen species. At the same time, the Zn 2p peaks showed a shift in doped samples, such as La DZ NPs at 1022.94 and 1047.37 eV, Sm DZ NPs at 1023.17 and 1046.42 eV, Sr DZ NPs at 1022.16 and 1045.36 eV in Sm, La, Sr TDZ NPs centered at 1022.73 and 1045.68 eV Fig. 5b. The presence of Zn+2 in oxide form is confirmed by an energy difference between spectral lines of 2p1/2 and 2p3/2 that agrees well with the standard value of 22.97 eV [37]. The O 1s region explained the different states and displayed two peaks as adsorption of the hydroxyl ion (OH−) or aqueous species over the surface of ZnO and oxygen vacancies. The first peak displayed a higher binding energy and showed at 536.43, 534.29, 532.22, 532.67, and 532.70 eV for PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, Sm, La, Sr TDZ NPs, respectively. In addition, the lower binding energy related to the oxygen vacancies demonstrated that oxygen vacancies are present at 533.10, 531.72, 531.9, 531.48, and 531.66 eV for PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, Sm, La, Sr TDZ NPs, respectively [29]. Arise new peaks correlate to La metal-doped observed in Figs. 6d and 9d, the binding energy of La 3d for single doped is located at 835.68 and 852.58 eV, while tri-doped appears at peaks 836.76 and 853.52 eV. Moreover, the La 3d showed that the La element existed as a (3 +) valence in the sample, according to the principle method and XPS instrument's manual [44]. Secondly, the Sm metal doped showed a peak in Fig. 7d for a single doped centered at 1082.34 eV. Surprisingly, the third Sr metal doped divided into two peaks for a single doped shown in Fig. 8d centered at 130.45 and 134.59 eV, assignable to 3d 5/2, and 3d 3/2 states, respectively. Similarly, the Sr in tri-doped showed two peaks at 130.85 and 135.22 eV Fig. 9f. These results exhibited that Sm3+, La3+, and Sr2+ ions are successfully incorporated into the ZnO lattice [45].
EM, TEM and EDX spectra
The surface morphology of PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs was studied using SEM and TEM analysis. Each scanning electron micrograph has the same magnification and a scale of 20 µ, clearly showing hexagonal ZnO and rod-shaped crystals [46, 47]. The hexagonal shapes don’t change due to uniform disperses of Sm3+, La3+, and Sr2+ ions across the hexagonal lattice (Fig. 10). In addition, the EDX spectra exhibited significant peaks for each Sm, La, and Sr metal, indicating that Sm3+, La3+, and Sr2+ ions are dispersed into the ZnO crystals lattice, according to our experimental measurements (Fig. 11). Additionally, the TEM images of PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, and Sr TDZ NPs showed that the ZnO particles are grown as a rod shape and have average particles size around 10–100 nm as shown in Fig. 12. Finally, the SEM and TEM images confirmed the successfully incorporating of Sm3+, La3+, and Sr2+ ions into the ZnO lattice.
Zeta potential and point of zero charge (pHpzc)
It is important to assess the stability of nanomaterials, where the suspension's net electrostatic potential is measured using its zeta potential. The PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, and Sr TDZ NPs had high positive potential values of 17.2, 13.0, 10.1, 13.9, and 10.6 mV, respectively (Fig. 13). In addition, the significant electrostatic repulsion between the values proved to be extremely stable and prevented the values from aggregating. Good nanoparticle stability is often assumed to occur at zeta potential levels between + 30 mV and −30 mV, as reported in the literature [44, 45]. Due to electrostatic attraction, the reactive dye is attracted to the positively charged surface of our doped nanomaterials, resulting in the adsorption of the reactive dye on the nanomaterials' surface.
Similarly, the point of zero charge (pHpzc) of PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs were determined by using a salt addition procedure. The adsorbent was added into sodium nitrate solution (50 mL) using both HCl (0.1 M) and NaOH (0.1 M) to maintain a pH range of 2 to 12, and after 24h of shaking at room temperature, and the final pH (pHf) was determined by pH meter. The point of zero charge (pHpzc) of nanomaterials was determined by plotting the graph between initial pHo & ΔpH (pHo – pHf). Additionally, the synthesized nanomaterials PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs showed values of 8.4, 8.2, 8.9, 8.7, and 9.2, respectively (Fig. 14). The surface of nanomaterials has a balance between positive and negative charges at a specific pH, which is denoted as pHpzc. Therefore, if the pH is less than pHpzc, the Nanoparticle surface will be positively charged, and if the pH is more than pHpzc, the surface will have a net negative charge [46]. Finally, it can be concluded that the values Low than pHpzc, the surface of nanomaterials became positive, and therefore, the electrostatic attraction occurred with reactive red 43.
Surface area
The BET method was used to estimate the pore diameter distribution and specific surface area of PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs (Fig. 15). The BET surface area is also a crucial metric for evaluating metal oxide. Comparing the PZ (SBET = 28.81 m2/g) with Sr DZ NPs (SBET = 37.43 m2/g) showed an increase in BET value. The increased BET surface area of Sr DZ NPs means that reactive dye molecules have a greater chance of adsorption with the Sr DZ NPs [47]. At the same time, La DZ NPs (SBET = 22.40 m2/g), Sm DZ NPs (SBET = 16.79 m2/g), and Sm, La, Sr TDZ NPs (SBET = 6.30 m2/g) exhibited a reduction of the BET surface area value. As a result, surface population density increased proportionally, resulting in a reduction in surface area [48]. Accordingly, the observed specific surface area may be linked to the large pore structure and external area as intra-particle pores, indicating a readily accessible area of both materials. The estimated pore size distribution pattern of the nanomaterials is shown in Fig. 15 (inset). Moreover, the overall pore volume of PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs was evaluated at 0.128, 0.1219, 0.1004, 0.2105, and 0.0277 cm3/g of each average pore size of 17.77, 21.82, 23.57, 22.50 and 17.572 nm, respectively. The addition of La and Sm metal led to a decrease in total pore volume compared to Sr, tri-doped sample [49].
Theoretical study
Crystal structures
The optimized geometry structure of a 2 × 2 × 2 supercell of PZ, La DZ NPs, and Sr DZ NPs, with 32 atoms, is constructed. Choose one metal from the alkaline-earth metals group and another metal from earth metals with different electronic configurations as an example of theoretical study. In ZnO, La atoms are substituted for Zn atoms to create La DZ NPs. In ZnO, Sr atoms are substituted for Zn atoms to create Sr DZ NPs, as illustrated in Fig. 16. The lattice parameters of the supercell models are shown in Table 1 and exhibited an increase in the lattice constants after doping. The theoretical results agree with the experimental results.
Electronic structure
We have estimated the band structure and the partial and total density of states for PZ, La DZ NPs, and Sr DZ NPs to learn more about their electrical structure. We operated the functional generalized gradient approximation (GGA + U) with the Perdew-Burke-Ernzerh (PBE) function, the result of the structure, and plotted gap energy shown in Fig. 17. These data demonstrate the existence of a direct band gap in the system, the value of which varies with the doping type. For PZ, the direct energy gap offered a value is 2.73 eV, proximate to the experimental value of 2.86 eV. In doped ZnO, the Fermi level shifted toward the valence band, which increases the electron carrier concentration of the band and promotes the creation of p-type semiconductors and system conductivity [50]. The calculated 2.77 and 2.85 eV gaps for La DZ NPs and Sr DZ NPs, respectively. These values agree with experimental results. The Burstein-Mott effect is responsible for this widening of the gap [51]. These values fit well with experimental data are 2.89 eV for La DZ NPs and 2.98 for Sr DZ NPs. To explain the formation of these band structures, we computed the total density of states (DOS) and partial density of state (PDOS) for PZ, La DZ, and Sr DZ NPs, as shown in Fig. 18. First, for PZ, the contribution of the O 2s states and Zn 3d states produced the first peak at around 14.89 eV in the valence band. O 2p and Zn 3d states predominate in the higher energy area of the valence band (between 7.41 and 0 eV). States of O 2p make up the top of the valence band. A strong Zn–O bond produced by the high interactions between the Zn 3d state and the 2p state of O, which range from 7.41 to 0 eV. The oxygen 2p, zinc 4p, and zinc 4s also control the bottom of the conduction band. Second, the O 2s, Zn 3d, and La 5p states are the origin of the first valence band peak for La DZ NPs, which are seen at 14.65 eV. O 2p, Zn 3d, and La 4d states dominate the lower valence band, which occupies the energy range from 7.27 to 0 eV, indicating that the orbital bonding relationship was strengthened. The conduction band is dominated by O 2p, Zn 4s, Zn 4p, La 4d, and La5p states. In doped ZnO, the Fermi level moves into the valence band, resulting in a p-type semiconductor. Third, O Zn 3d, and Sr 4p states contribute to the lowest peak at − 14.77 eV in Sr-doped ZnO. The energy range between -7.35 and 0 eV and the energy band toward the top of the valence band are dominated by the O 2p, Zn 3d, and Sr 3d states. Valence band bandwidth increases due to O2p-Sr 3d state interaction. The conduction band widens when electronic states overlap. Doped systems have lower densities of states than PZ, and the Fermi level advances into the valence band, increasing the width of both the conduction and valence bands and the band gap compared to PZ [52, 53].
Optical properties
Density functional theory computed optical characteristics, such as dielectric function. The dielectric function in crystals is the link between electronic transition and electronic structure, reflecting a certain material's band structure and optical properties. The optical properties of semiconductor materials can be described by complex dielectric functions, according to solid state theory. Calculating inertia matrix elements of occupied and non-occupied states and Kramers–Kronig dispersion relations yields dielectric function real and imaginary parts [54, 55]. The electron transition between conduction and valence bands determines the imaginary part. For any material, the imaginary part of the dielectric function is significant. The imaginary part connects the inter-band transition micro-physical process to the solid electronic structure, reflecting the solid energy band structure and other spectrum information. Figure 19 shows the imaginary and real dielectric functions of PZ, La DZ, and Sr DZ NPs. The PZ's imaginary dielectric function exhibits three high peaks (8.82, 12.28, and 14.55 eV) that match other theoretical conclusions [56]. Three peaks may be identified: the first is obtained from the transition between O-2p and Zn-4s, the second from the transition between Zn-3d and O-2p, and the third from the transition between Zn-3d and O-2s. According to the investigation of the density of states and band structure, the electron transition of 4d states for the doped ZnO exhibits a pear peak at about 4.86 eV. Doping causes a slight drop in dielectric constant in the low-energy range.
Study of photocatalytic activity
RR43 dye degradation was studied in pure and doped ZnO under UV light irradiation to find the best photocatalytic conditions. RR43 dye is extremely stable in water. The degradation of RR43 dye with PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, and Sm, La, Sr TDZ NPs within constant reaction time from 0 to 120 min, where 0 min in each case is the dark study that is before irradiation with UV light. It has been noticed that Sr DZ NPs exhibited higher photocatalytic degradation at 93.43% Then, PZ at 72.88%, La DZ NPs at 52.54%, Sm DZ NPs at 31.99%, and La, Sm, Sr T DZ NPs 20.55%. On the surface, the Sr2+ ions in the lattice act as electron scavengers, preventing the recombination of electron–hole pairs and extending their lifetime, and can capture photo-induced electrons, slowing electron–hole recombination, resulting in a decrease in recombination rate due to increased defects that develop enhancement of photocatalytic degradation [57]. The degradation is confirmed as the absorbance of dye, and absorbance decreases strongly with Sr DZ NPs, then La DZ NPs, Sm DZ NPs finally Tri-doped. The fastest and most efficient degradation was observed by Sr DZ NPs due to Sr, one of the alkaline-earth metals, which this group considers as the second most reactive family of elements related to electronic configuration.
Effect of photocatalytic dose
An important variable in photodegradation is photocatalytic dosage. We have investigated the effect of photocatalytic dosage on the photodegradation percentage of RR43. The experimental results were performed at 25 °C with UV irradiation using varying concentrations of photocatalytic nanomaterials (0.00, 0.02, 0.05, and 0.07 g/ L) and a constant concentration of RR43 dye (20 ppm/50 mL). As represented in Fig. 20, the photodegradation % increased with increasing adsorbent dosage due to an increase in photocatalytic available active surface area [57].
Effect of dye concentration
The photodegradation investigation of RR43 was carried out in the presence of 0.05 g/L of photocatalytic nanomaterials under UV light irradiation at various dye solution concentrations (10, 20, 30, and 40 ppm). Figure 21 showed the photodegradation of dye solution concentrations under the same conditions for all photocatalytic nanomaterials. In this study, the Beer-Lambert rule may explain the decrease in photodegradation percentage as the RR43 dye concentration increased, where more dye molecules were absorbed to the surface of the photocatalytic nanomaterials. A reduced photodegradation activity may result from a reduced path length for light entering the solution as dye concentration increases. Furthermore, an increase in the initial concentration of RR43 dye may cause a decrease in the production of OH radicals on the surface, resulting in the RR43 molecules blocking the active sites. [33].
Effect of solution pH
The effect of solution pH on photocatalytic degradation of RR43 was examined using initial pH values of the aqueous RR43 solution of 3, 5.8, 8, 10, and 12 adjusted by aqueous 0.1M HCl and 0.1M NaOH solutions while keeping all other experimental conditions constant. It was demonstrated that photodegradation depends on dye adsorption on the photocatalytic surface and that dye adsorption is greatly influenced by the pH of the solution. Figure 22 depicts the influence of the initial pH on RR43 degradation. These results were confirmed by measuring the zero-point charge (pHpzc), determined at a pH higher than 8. It was noted that the best degradation percentage of RR43 at pH 8 was recorded at 93.43% for Sr DZ NPs, 72.88% for PZ, 52.54% for La DZ NPs, 31.99% for Sm DZ NPs, and 20.55% for La, Sm, Sr T DZ NPs. The values at pH 8 consider value Low than pHpzc, so the photocatalytic surface will become a positive charge, enhancing electrostatic attraction with reactive red 43 [33]. The values of degradation percentage at pH 10 and 12 were recorded (70.34% and 4.03%) for Sr DZ NPs, (48.73% and 12.71%) for PZ, (25.42% and 6.56%) for La DZ NPs, (11.44% and 9.11%) for Sm DZ NPs and, (11.02% and 9.32%) for La, Sm, Sr T DZ NPs. This decrease in the degradation percentage at pH 10 and 12 may be related to the fact that the surface of the photocatalytic will become a negative charge, which is repulsive with RR43. Finally, at a pH3, the photodegradation percentage decrease showed 82.84% for Sr DZ NPs, 52.11% for PZ, 45.97% for La DZ NPs, 24.15% for Sm DZ NPs, and 13.56% for La, Sm, Sr T DZ NPs, due to Nanoparticle breakdown in acidic pH.
Adsorption kinetic studies
Kinetic studies were carried out using pseudo-first-order models [58] using experimental data (Eq. 3) defines the pseudo-first-order kinetic model:
where C represents the dye solution intensity after irradiation, Co represents the preliminary dye solution intensity before irradiation, t represents the time (min), and k (min−1) represents the pseudo-first-order rate constant. The fitting curves were plotted ln(C/Co) versus time [59], which exhibited that the degradation of RR43 dye roughly obeys pseudo-first-order kinetics. We found that the R2 values obtained from the kinetic model offer that the photocatalytic nanomaterials are more fitted with the pseudo-First-order kinetic model due to the higher R2 quantities. The pseudo-first-order rate constant (k) for all photocatalytic nanomaterials with degradation of RR43 recorded a higher value14.84 *10–3(min−1) with Sr DZ NPs, then PZ 7.07 *10–3(min−1), La DZ NPs 3.85 *10–3(min−1), Sm DZ NPs 2.13 *10–3(min−1), and La, Sm, Sr T DZ NPs 1.31*10–3(min−1) shown Fig. 23.
Mechanism of photocatalytic degradation of reactive Red 34
The Sr2+ dopant modifications ZnO, which traps excited electrons in the CB and reduces electron hole-recombination. Through a series of reactions, the electrons trapped by dopants would finally react with ˙O2 to create [˙OH]. Through a series of reactions, the electrons trapped by dopants would finally react with O2 to create [˙OH]. The H2O molecule or [˙OH] adsorbed on the photocatalytic surface would be charged up by the hole in the valence band of doped ZnO, forming [˙OH]. The generated [˙OH] would react with RR43 dye molecules adsorbed on the surface during photocatalytic to destroy the pollutants. Second, rare earth reacts with the electrons in the conduction band when La3+ and Sm3+ are used as dopants. This is because rare earth metals are forceful Lewis’s acids with a partially filled 4f orbital that can trap the electron and further prevent the recombination of charge carriers. Unstable Sm2+ ions lost electrons to oxygen molecules to create active superoxide radicals, which protonated to ˙OOH radicals. The inactive OOH radical is converted into the reactive OH radical. The generation of [˙OH] in doped ZnO can be explained as follows: increasing the optical band gap value keeps photo-generated electrons in the conduction band, enhancing the material's photocatalytic action. In addition, the dopant of La, Sm, and Sr atoms will create intermediate energy levels in the doped lattice, where photo-excited electrons will trap. Additionally, the PL spectrum confirmed that the electron–hole recombination rate was reduced. Scheme 1 depicts the degradation pathway schematically.
Recyclability of the photocatalytic nanomaterials
Recyclability and stability are important parameters for assessing the effectiveness of a photocatalytic process since they allow the photocatalytic to recycle repeatedly for pollutant degradation [60]. Repeating the photocatalytic experiment four times under the same conditions, with the photocatalyst separated from the dye solution via centrifugation after each cycle run, and then using it again after washing and drying, allowed us to evaluate the recyclability of the photocatalytic nanomaterials [33]. After four cycle runs, La, Sm, Sr T DZ, La DZ NPs, and Sm DZ NPs, these compounds showed the photodegradation percentage decreased slightly than Sr DZ NPs and PZ. So, consider La, Sm, Sr T DZ, La DZ NPs, and Sm DZ NPs are more stable due to having rare earth metals. Additionally, shown in Fig. 24, the photodegradation percentage decreases slightly, and the dye degradation is consistent. This reduction could be because photocatalytic is lost when the sample is washed; the photocatalytic nanomaterials showed stability from this result. This current study significantly compares to previously reported studies on degeneration efficiency (Table 2).
Conclusions
In this work, new nanomaterials such as pure zinc oxide, single doped ZnO nanomaterials (2%), namely as Sm DZ NPs, La DZ NPs, Sr DZ NPs, and tri-doped ZnO as Sm, La, Sr, T DZ NPs were synthesized using hydrothermal method. The structure of the synthesized nanomaterials based on ZnO (single and tri-doped) was confirmed using different spectroscopic techniques, such as X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), ultraviolet (UV) light, photoluminescence (PL), scanning electron microscopy (SEM) and energy-dispersive X-ray (XPS), transmission electron microscopy (TEM), and the point of zero charges (pHpzc). The XRD confirmed a hexagonal crystal structure without change in the crystalline phase after doping and exhibited an increase in the average crystallite size. The XRD pattern displayed a shift in peaks and changes in lattice characteristics, indicating that Sm3+, La3+, and Sr2+ ions are replacing Zn2+ ions. The FT-IR spectroscopy showed shifting in the absorption of Zn–O band at 455.14, 512.285, 465.98, and 514.93 cm−1 for La DZ NPs, Sm DZ NPs, Sr DZ NP, and Sm, La, Sr TDZ NPs, respectively, compared to pure ZnO 464.63 cm−1. Moreover, using UV–Vis measurements and the Tauc equation, the energy band gap values were measured experimentally (2.86, 2.89, and 2.98 eV) and calculated theoretically by DFT calculation to afford values of (2.73, 2.77, and 2.85 eV) for PZ, La DZ NPs, Sm DZ NPs, Sr DZ NP, and Sm, La, Sr TDZ NPs, respectively. The theoretical result confirmed the increase in the band gap due to an increase in the atomic radius of the dopant. The PL spectrum displays showed peaks at nearly 470 nm related to the high density of oxygen vacancies. The morphology study (SEM and TEM) showed hexagonal and rod-shaped crystals that do not change with doped nanomaterials. Furthermore, XPS and EDX data exhibited significant peaks for each Sm, La, and Sr metal, indicating that Sm3+, La3+, and Sr2+ ions are dispersed into the ZnO crystals lattice. The Zeta potential demonstrated high positive potential values of 17.2, 13.0, 10.1, 13.9, and 10.6 mV for PZ, La DZ NPs, Sm DZ NPs, Sr DZ NPs, respectively. The BET surface area is also evaluated, and Sr DZ NPs recorded a high value (SBET = 37.43 m2/g). Additionally, the photodegradation of Reactive Red 43 (RR43) dye was studied using different factors, such as photocatalyst dose, dye concentration, and pH. The results revealed that the Sr DZ NPs showed the best photodegradation percentage (93.43%) compared to PZ (72.88%), La DZ NPs (52.54 3%), Sm DZ NPs (31.99%), and La, Sm, Sr T DZ NPs (20.55%). The kinetic studies exhibited that the R2 values are more fitted with the pseudo-first-order kinetic model, and cyclic stability proves the suitability of synthesized nanomaterials under visible light irradiation for the treating of the RR43 dye as polluted wastewaters.
Data availability
All the datasets used and/or analyzed in this study are available in the manuscript and supplementary information can be asked from the corresponding author upon request.
References
Muraro PCL, Mortari SR, Vizzotto BS et al (2020) Iron oxide nanocatalyst with titanium and silver nanoparticles: synthesis, characterization and photocatalytic activity on the degradation of Rhodamine B dye. Sci Rep 10:3055. https://doi.org/10.1038/s41598-020-59987-0
Bhakya S, Muthukrishnan S, Sukumaran M et al (2015) Catalytic degradation of organic dyes using synthesized silver nanoparticles: a green approach. J Bioremed Biodeg. https://doi.org/10.4172/2155-6199.1000312
Deng L, Geng M, Zhu D et al (2012) Effect of chemical and biological degumming on the adsorption of heavy metal by cellulose xanthogenates prepared from Eichhornia crassipes. Bioresour Technol 107:41–45. https://doi.org/10.1016/j.biortech.2011.12.023
Mohammadi R, Tang W, Sillanpää M (2021) A systematic review and statistical analysis of nutrient recovery from municipal wastewater by electrodialysis. Desalination 498:114626. https://doi.org/10.1016/j.desal.2020.114626
Emam AA, Abo Faraha SA, Kamal FH et al (2020) Modification and characterization of Nano cellulose crystalline from Eichhornia crassipes using citric acid: an adsorption study. Carbohydr Polym 240:116202. https://doi.org/10.1016/j.carbpol.2020.116202
Naseem S, Wu C, Motora KG (2021) Novel multifunctional Rb x WO 3 @ Fe 3 O 4 immobilized Janus membranes for desalination and synergic-photocatalytic water purification. Desalination 517:115256. https://doi.org/10.1016/j.desal.2021.115256
Zhang D, Zhang M, Chen S et al (2021) Scalable, self-cleaning and self-floating bi-layered bacterial cellulose biofoam for efficient solar evaporator with photocatalytic purification. Desalination 500:114899. https://doi.org/10.1016/j.desal.2020.114899
Kumar P, Chand P (2022) Large electric field driven strain and enhanced multiferroic properties of Ce3+/BiFeO3Nano photo-catalyst. Mater Sci Semicond Process 144:106576. https://doi.org/10.1016/j.mssp.2022.106576
Tanwar R, Kaur B, Kumar Mandal U (2017) Highly efficient and visible light driven Ni0.5Zn0.5Fe2O4@PANI modified BiOCl heterocomposite catalyst for water remediation. Appl Catal B Environ 211:305–322. https://doi.org/10.1016/j.apcatb.2017.04.051
Zhu X, Blanco E, Bhatti M, Borrion A (2020). J Pre Sci Total Environ. https://doi.org/10.1016/j.chemosphere.2023.138322
Kumar P, Chand P (2022) Sm3+-BiFeO3 nano catalyst: a synergetic effect of Sm3+ on enhanced multiferroic properties and photocatalysis. J Alloys Compd 891:161896. https://doi.org/10.1016/j.jallcom.2021.161896
Kumar P, Chand P (2022) Electric field-driven energy storage density and photo-catalytic temperament of Gd3+-BiFeO3 nano-ferrite. J Mater Sci Mater Electron 33:2980–2994. https://doi.org/10.1007/s10854-021-07498-7
Sukriti PC (2020) Optical and photocatalytic response to solvent-based synthesis of SnO2 nanostructures. Mater Today Proc 28:188–192. https://doi.org/10.1016/j.matpr.2020.01.542
Sukriti PC, Singh V, Kumar D (2020) Rapid visible light-driven photocatalytic degradation using Ce-doped ZnO nanocatalysts. Vacuum 178:109364. https://doi.org/10.1016/j.vacuum.2020.109364
Saddik MS, Elsayed MMA, El-Mokhtar MA et al (2022) Tailoring of novel azithromycin-loaded zinc oxide nanoparticles for wound healing. Pharmaceutics 14:111. https://doi.org/10.3390/pharmaceutics14010111
Khan MM, Adil SF, Al-Mayouf A (2015) Metal oxides as photocatalysts. J Saudi Chem Soc 19:462–464
Mohamed WS, Abu-Dief AM (2018) Synthesis, characterization and photocatalysis enhancement of Eu2O3-ZnO mixed oxide nanoparticles. J Phys Chem Solids 116:375–385. https://doi.org/10.1016/j.jpcs.2018.02.008
Yi H, Liu R, Chen Z, Nie B (2020) Visible-light driven photodegradation on Ag nanoparticle-embedded fullerene (C60) heterostructural microcubes. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.127355
Okur HE, Bulut N, Ates T, Kaygili O (2019) Structural and optical characterization of Sm-doped ZnO nanoparticles. Bull Mater Sci 42:1–9. https://doi.org/10.1007/s12034-019-1877-2
Kafle BP (2020) Chapter 6 - Introduction to nanomaterials and application of UV–Visible spectroscopy for their characterization. In: Kafle BPBT-CA and MC by S (ed). Elsevier, pp 147–198
Ekthammathat N, Thongtem T, Phuruangrat A, Thongtem S (2013) Photoluminescence of hexagonal ZnO nanorods hydrothermally grown on Zn foils in KOH solutions with different values of basicity. J Nanomater 2023:208230. https://doi.org/10.1155/2013/208230
Gan YX, Jayatissa AH, Yu Z et al (2020) Hydrothermal synthesis of nanomaterials. J Nanomater 2020:8917013. https://doi.org/10.1155/2020/8917013
Ejsmont A, Goscianska J (2023) Hydrothermal synthesis of ZnO superstructures with controlled morphology via temperature and PH optimization. Materials (Basel) 16:1641
Li Y, Yu Y, Wang H, Zhao F (2016) Effect of process parameters on the recrystallization and size control of puerarin using the supercritical fluid antisolvent process. Asian J Pharm Sci 11:281–291. https://doi.org/10.1016/j.ajps.2015.12.001
Kumar B, Kumar S, Singh V et al (2020) Preparation of strontium doped mesoporous ZnO nanoparticles to investigate their dye degradation efficiency. Nano Express. https://doi.org/10.1088/2632-959X/abc393
Yousefi R, Jamali-Sheini F, Cheraghizade M et al (2015) Enhanced visible-light photocatalytic activity of strontium-doped zinc oxide nanoparticles. Mater Sci Semicond Process 32:152–159. https://doi.org/10.1016/j.mssp.2015.01.013
Hernández-Carrillo MA, Torres-Ricárdez R, García-Mendoza MF et al (2020) Eu-modified ZnO nanoparticles for applications in photocatalysis. Catal Today 349:191–197. https://doi.org/10.1016/j.cattod.2018.04.060
Ravichandran AT, Karthick R, Xavier AR et al (2017) Influence of Sm doped ZnO nanoparticles with enhanced photoluminescence and antibacterial efficiency. J Mater Sci Mater Electron 28:6643–6648. https://doi.org/10.1007/s10854-017-6355-2
Baskaran P, Pramothkumar A, Mani P (2022) Enhanced photo catalytic activity of ZnO nano particles Co-doped with rare earth elements (Nd and Sm) under UV light illumination. J Inorg Organomet Polym Mater 32:1668–1680. https://doi.org/10.1007/s10904-022-02228-w
Porkalai V, Anburaj DB, Sathya B et al (2017) Study on the synthesis, structural, optical and electrical properties of ZnO and lanthanum doped ZnO nano particles by sol-gel method. Mech Mater Sci Eng. https://doi.org/10.2412/mmse.77.37.393
Rafique M, Tahir R, Bilal NRKM et al (2021) Hydrothermal synthesis of an efficient and visible light responsive pure and strontium doped zinc oxide nano - hexagonal photocatalysts for photodegradation of Rhodamine B dye. Appl Nanosci 11:1045–1056. https://doi.org/10.1007/s13204-021-01669-y
Manikandan A, Manikandan E, Meenatchi B et al (2017) Rare earth element (REE) lanthanum doped zinc oxide (La: ZnO) nanomaterials: synthesis structural optical and antibacterial studies. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2017.06.336
Faraz M, Naqvi FK, Shakir M, Khare N (2018) Synthesis of samarium-doped zinc oxide nanoparticles with improved photocatalytic performance and recyclability under visible light irradiation. New J Chem 42:2295–2305. https://doi.org/10.1039/c7nj03927a
Kumar DR, Ranjith KS, Nivedita LR, Kumar RTR (2017) Effect of samarium doping on structural, optical and magnetic properties of vertically aligned ZnO nanorod arrays. J Rare Earths 35:1002–1007. https://doi.org/10.1016/S1002-0721(17)61005-6
Perumal SL, Hemalatha P, Alagara M, Pandiyaraj KN (2015) Investigation of structural, optical and photocatalytic properties of Sr doped Zno nanoparticles. Int J Phys Sci 4:1–13
Prasad R, Walke PS (2019) Structural and optical studies on flowerlike strontium doped zinc oxide synthesized by hydrothermal method. Mater Res Express. https://doi.org/10.1088/2053-1591/ab4deb
Thool GS, Singh AK, Singh SP (2015) Shape tunable synthesis of Eu- and Sm-doped ZnO microstructures: a morphological evaluation. Bull Mater Sci 38:1519–1525. https://doi.org/10.1007/s12034-015-0967-z
Pascariu P, Cojocaru C, Olaru N et al (2019) Novel rare earth (RE-La, Er, Sm) metal doped ZnO photocatalysts for degradation of Congo-Red dye: synthesis, characterization and kinetic studies. J Environ Manage 239:225–234. https://doi.org/10.1016/j.jenvman.2019.03.060
Almoussawi M, Abdallah AM, Habanjar K, Awad R (2020) Effect of (Sm, Co) co-doping on the structure and electrical conductivity of ZnO nanoparticles. Mater Res Express. https://doi.org/10.1088/2053-1591/abbfbd
Etacheri V, Roshan R, Kumar V (2012) Mg-doped ZnO nanoparticles for efficient sunlight-driven photocatalysis. ACS Appl Mater Interfaces 4:2717–2725
Chand P, Singh V (2020) Enhanced visible-light photocatalytic activity of samarium-doped zinc oxide nanostructures. J Rare Earths 38:29–38. https://doi.org/10.1016/j.jre.2019.02.009
Salaya‐Gerónimo E, García‐Zaleta DS, Jácome‐Acatitla G et al (2022) Structural, optical and photocatalytic properties of Sr doped and Ca doped BiFeO3 compounds prepared by Pechini method. J Chem Technol Biotechnol 97:2970–2983. https://doi.org/10.1002/jctb.7084
Mohite RM, Ansari JN, Roy AS, Kothawale RR (2016) Significant enhancement in the conductivity of Al-doped zinc oxide thin films for TCO application. Int J Nanosci 15:1650011
Al-Kordy HMH, Sabry SA, Mabrouk MEM (2021) Statistical optimization of experimental parameters for extracellular synthesis of zinc oxide nanoparticles by a novel haloalaliphilic Alkalibacillus sp.W7. Sci Rep 11:1–14. https://doi.org/10.1038/s41598-021-90408-y
Selvarajan E, Mohanasrinivasan V (2013) Biosynthesis and characterization of ZnO nanoparticles using Lactobacillus plantarum VITES07. Mater Lett 112:180–182. https://doi.org/10.1016/j.matlet.2013.09.020
Leiva E, Tapia C, Rodr C (2021) Highly Efficient Removal of Cu ( II ) Ions from Acidic Aqueous Solution Using ZnO Nanoparticles as Nano-Adsorbents. MDPI 1–21
Zhang YH, Peng MX, Yue LJ et al (2021) A room-temperature aniline sensor based on Ce doped ZnO porous nanosheets with abundant oxygen vacancies. J Alloys Compd 885:160988. https://doi.org/10.1016/j.jallcom.2021.160988
Anucha CB, Bacaksiz E, Stathopoulos VN et al (2022) Preparation and characterization of supported molybdenum doped TiO2 on α-Al2O3 ceramic substrate for the photocatalytic degradation of ibuprofen (IBU) under UV irradiation. Catalysts 12:562
Salmas CE, Stathopoulos VN, Pomonis PJ, Androutsopoulos GP (2002) Pore structure-chemical composition interactions of new high surface area manganese based mesoporous materials. Materials preparation, characterization, and catalytic activity. Langmuir 18:423–432. https://doi.org/10.1021/la010340d
da Fonseca AFV, Siqueira RL, Landers R et al (2018) A theoretical and experimental investigation of Eu-doped ZnO nanorods and its application on dye sensitized solar cells. J Alloys Compd 739:939–947
Gahlawat S, Singh J, Yadav AK, Ingole PP (2019) Exploring Burstein-Moss type effects in nickel doped hematite dendrite nanostructures for enhanced photo-electrochemical water splitting. Phys Chem Chem Phys 21:20463–20477
Zhang R, Zhao J, Yang Y et al (2016) Understanding electronic and optical properties of La and Mn co-doped anatase TiO2. Comput Condens Matter 6:5–17. https://doi.org/10.1016/j.cocom.2016.03.001
Islam MR, Saiduzzaman M, Nishat SS et al (2021) Synthesis, characterization and visible light-responsive photocatalysis properties of Ce doped CuO nanoparticles: a combined experimental and DFT+U study. Colloids Surf A Physicochem Eng Asp 617:126386. https://doi.org/10.1016/j.colsurfa.2021.126386
Al-Bediry S, Taghavi Mendi R, Bakhshayeshi A (2021) Investigation of structural, electronic, and optical properties of Si0. 67Ge0. 33 alloy: a DFT approach. Opt Quantum Electron 53:1–9
Huang X, Wu Q, Dai R et al (2022) A density functional study of the structural, electronic, optical and lattice dynamical properties of AgGaS2. Results Phys 35:105309. https://doi.org/10.1016/j.rinp.2022.105309
Borovac D, Tan C-K, Tansu N (2018) First-principle study of the optical properties of dilute-P GaN1− xPx alloys. Sci Rep 8:1–9
Khataee A, Kayan B, Gholami P et al (2017) Sonocatalytic degradation of an anthraquinone dye using TiO2-biochar nanocomposite. Ultrason Sonochem 39:120–128. https://doi.org/10.1016/j.ultsonch.2017.04.018
Elmolla ES, Chaudhuri M (2010) Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV / TiO 2 and UV / H 2 O 2 / TiO 2 photocatalysis. Desalin J 252:46–52. https://doi.org/10.1016/j.desal.2009.11.003
Zhang W, Li Y, Wang C, Wang P (2011) Kinetics of heterogeneous photocatalytic degradation of rhodamine B by TiO 2 -coated activated carbon: roles of TiO 2 content and light intensity. Desalination 266:40–45. https://doi.org/10.1016/j.desal.2010.07.066
Tahir N, Zahid M, Bhatti IA, Jamil Y (2022) Fabrication of visible light active Mn-doped Bi2WO6-GO/MoS2 heterostructure for enhanced photocatalytic degradation of methylene blue. Environ Sci Pollut Res 29:6552–6567. https://doi.org/10.1007/s11356-021-16094-5
Akram R, Fatima A, Almohaimeed ZM et al (2023) Photocatalytic degradation of methyl green dye mediated by pure and Mn-doped zinc oxide nanoparticles under solar light irradiation. Adsorpt Sci Technol 2023:5069872. https://doi.org/10.1155/2023/5069872
Devi KN, Devi SA, Singh WJ, Singh KJ (2021) Nickel doped zinc oxide with improved photocatalytic activity for Malachite Green Dye degradation and parameters affecting the degradation. J Mater Sci Mater Electron 32:8733–8745
Kumar B, Kumar S, Singh V et al (2020) Preparation of strontium doped mesoporous ZnO nanoparticles to investigate their dye degradation efficiency. Nano Express 1:30015
Acknowledgements
This paper is based upon work supported by science, Technology, Innovation Funding Authority (STDF) under grant. STDF Call 1/Post Graduate Support Grant (PGSG) Project (No: 44771).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MB Data curation, visualization, investigation, writing—original draft. AE Conceptualization, project administration, resources, supervision, writing—review & editing, methodology, validation. FK, AG, and SAF: visualization, investigation, validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Handling Editor: Andrea de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Basseem, M., Emam, A.A., Kamal, F.H. et al. Novel functionalized of ZnO with Sm3+, La3+, and Sr2+/ZnO single and tri-doped nanomaterials for photocatalytic degradation: synthesis, DFT, kinetics. J Mater Sci 58, 13346–13372 (2023). https://doi.org/10.1007/s10853-023-08829-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08829-1