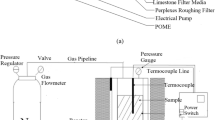
Abstract
This work reports the preparation and application of a microfiltration membrane based on kaolin. The membrane is characterized using various techniques such as XRD, FESEM, porosity, pure water permeation, and chemical stability tests. The fabricated membrane with a pore size of 1.66 μm, porosity of 45.46%, and pure water permeability of 1.8 × 10−8 (m3 m−2 s−1 kPa−1) was utilized for studying iron removal from an aqueous solution by hybrid oxidation–microfiltration process. Response surface methodology (RSM) via Box–Behnken design (BBD) was used to study the effect of different input parameters (applied pressure, oxidant dosage, and initial concentration of iron) on permeate flux and iron removal. The experimental data were analysed using a model based on a second-order polynomial, which was then statistically confirmed. Analysis of variance (ANOVA) was used to develop and test the quadratic models between each response and the independent variables. The p value and F-values of the models developed for both the responses indicated the developed models were highly significant. The optimum conditions were found to be at an initial iron concentration of 49.95 mg L−1, an oxidant dose of 22.38 mg L−1, and applied pressure of 3.05 bar at which the permeate flux and rejection reported values of 1.32 × 10−6 m3 m−2 s−1 and 83.05%, respectively. These results agreed quite well with the experimental values. Based on these results the optimization of the microfiltration process using BBD was reliable in predicting the performance within the limits of the input parameters employed.







Similar content being viewed by others
References
Singh R (2015) Membrane technology and engineering for water purification, 2nd edn. Butterworth-Heinemann
Garg NK, Hassan Q (2007) Alarming scarcity of water in India. Curr Sci 93:932–941
Silver J (1993) Introduction to iron chemistry. In: Chemistry of iron. Springer Netherlands, Dordrecht.
Chaturvedi S, Dave PN (2012) Removal of iron for safe drinking water. Desalination 303:1–11. https://doi.org/10.1016/j.desal.2012.07.003
Khatri N, Tyagi S (2015) Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front Life Sci 8:23–39. https://doi.org/10.1080/21553769.2014.933716
bin Jusoh A, Cheng WH, Low WM et al (2005) Study on the removal of iron and manganese in groundwater by granular activated carbon. Desalination 182(1–3):347–353. https://doi.org/10.1016/j.desal.2005.03.022
Tekerlekopoulou AG, Pavlou S, Vayenas DV (2013) Removal of ammonium, iron and manganese from potable water in biofiltration units: a review. J Chem Technol Biotechnol 88:751–773. https://doi.org/10.1002/jctb.4031
Ellis D, Bouchard C, Lantagne G (2000) Removal of iron and manganese from groundwater by oxidation and microfiltration. Desalination 130:255–264. https://doi.org/10.1016/S0011-9164(00)00090-4
Samantha SK (2020) Ground water year book of West Bengal & Andaman & Nicobar Islands.
Kumar V, Bharti PK, Talwar M et al (2017) Studies on high iron content in water resources of Moradabad district (UP), India. Water Sci 31:44–51. https://doi.org/10.1016/j.wsj.2017.02.003
Mahanta DB, Das NN, Dutta RK (2004) A chemical and bacteriological study of drinking water in tea gardens of central Assam. Indian J Environ Prot 24:654–660
Kalita PJ, Gogoi C, Bhattacharyya SM, Goswamee RL (2021) Hydro chemical assessment of ground water in North-Eastern Region of India: a case study of western suburb of Jorhat town of Assam India. Curr World Environ 16:1–2. https://doi.org/10.12944/CWE.16.1.04
Rayees Ahmad Pir (2020) Ground water year book 2018–19 Jammu & Kashmir.
Zheng Q, Zhao Y, Guo J et al (2017) Iron overload promotes erythroid apoptosis through regulating HIF-1a/ROS signaling pathway in patients with myelodysplastic syndrome. Leuk Res 58:55–62. https://doi.org/10.1016/j.leukres.2017.04.005
Hartmann J, Braulke F, Sinzig U et al (2013) Iron overload impairs proliferation of erythroid progenitors cells (BFU-E) from patients with myelodysplastic syndromes. Leuk Res 37:327–332. https://doi.org/10.1016/j.leukres.2012.11.005
Chai X, Li D, Cao X et al (2015) ROS-mediated iron overload injures the hematopoiesis of bone marrow by damaging hematopoietic stem/progenitor cells in mice. Sci Rep 5:1–11. https://doi.org/10.1038/srep10181
Shao L, Li H, Pazhanisamy SK et al (2011) Reactive oxygen species and hematopoietic stem cell senescence. Int J Hematol 94:24–32. https://doi.org/10.1007/s12185-011-0872-1
Prus E, Fibach E (2010) Effect of iron chelators on labile iron and oxidative status of thalassaemic erythroid cells. Acta Haematol 123:14–20. https://doi.org/10.1159/000258958
Alimohammadi V, Sedighi M, Jabbari E (2017) Experimental study on efficient removal of total iron from wastewater using magnetic-modified multi-walled carbon nanotubes. Ecol Eng 102:90–97. https://doi.org/10.1016/j.ecoleng.2017.01.044
Das B, Hazarika P, Saikia G et al (2007) Removal of iron from groundwater by ash: a systematic study of a traditional method. J Hazard Mater 141:834–841. https://doi.org/10.1016/j.jhazmat.2006.07.052
Michalakos G (1997) Removal of iron from potable water using a trickling filter. Water Res 31:991–996. https://doi.org/10.1016/S0043-1354(96)00343-0
Phatai P, Wittayakun J, Chen W-H et al (2014) Removal of manganese(II) and iron(II) from synthetic groundwater using potassium permanganate. Desalination Water Treat 52:5942–5951. https://doi.org/10.1080/19443994.2013.819150
Du X, Liu G, Qu F et al (2017) Removal of iron, manganese and ammonia from groundwater using a PAC-MBR system: The anti-pollution ability, microbial population and membrane fouling. Desalination 403:97–106. https://doi.org/10.1016/j.desal.2016.03.002
Jasiewicz K, Pietrzak R (2013) The influence of pore generating agent on the efficiency of copper and iron ions removal from liquid phase by polyethersulfone membranes. Chem Eng J 228:449–454. https://doi.org/10.1016/j.cej.2013.05.005
Tekerlekopoulou AG, Vasiliadou IA, Vayenas DV (2006) Physico-chemical and biological iron removal from potable water. Biochem Eng J 31:74–83. https://doi.org/10.1016/j.bej.2006.05.020
Ghosh D, Solanki H, Purkait MK (2008) Removal of Fe(II) from tap water by electrocoagulation technique. J Hazard Mater 155:135–143. https://doi.org/10.1016/j.jhazmat.2007.11.042
Nandeshwar SN, Mahakalakar AS, Gupta RR, Kyzas GZ (2016) Green activated carbons from different waste materials for the removal of iron from real wastewater samples of Nag River. India J Mol Liq 216:668–692. https://doi.org/10.1016/j.molliq.2015.12.065
Nemade P, Kadam AM, Shankar HS (2008) Arsenic and iron removal from water using constructed soil filter-a novel approach. Asia-Pacific J Chem Eng 3:497–502. https://doi.org/10.1002/apj.173
Wang Y, Sikora S, Kim H et al (2013) Effects of solution chemistry on the removal reaction between calcium carbonate-based materials and Fe(II). Sci Total Environ 443:717–724. https://doi.org/10.1016/j.scitotenv.2012.11.009
Víctor-Ortega MD, Ochando-Pulido JM, Martínez-Ferez A (2016) Iron removal and reuse from Fenton-like pretreated olive mill wastewater with novel strong-acid cation exchange resin fixed-bed column. J Ind Eng Chem 36:298–305. https://doi.org/10.1016/j.jiec.2016.02.019
Shavandi MA, Haddadian Z, Ismail MHS et al (2012) Removal of Fe(III), Mn(II) and Zn(II) from palm oil mill effluent (POME) by natural zeolite. J Taiwan Inst Chem Eng 43:750–759. https://doi.org/10.1016/j.jtice.2012.02.014
Bordoloi S, Nath SK, Gogoi S, Dutta RK (2013) Arsenic and iron removal from groundwater by oxidation–coagulation at optimized pH: laboratory and field studies. J Hazard Mater 260:618–626. https://doi.org/10.1016/j.jhazmat.2013.06.017
Vigneswaran S, Visvanathan C (1995) Water treatment processes: Simple options. CRC Press, New York
R.B. Robinson, State-of the-Art: iron and manganese control, In: Proceedings of the New England water works association conference and exhibition. Marlborough, MA, 1998.s
Choo K, Lee H, Choi S (2005) Iron and manganese removal and membrane fouling during UF in conjunction with prechlorination for drinking water treatment. J Membr Sci 267:18–26. https://doi.org/10.1016/j.memsci.2005.05.021
Korchef A, Kerkeni I, Amor MB et al (2009) Iron removal from aqueous solution by oxidation, precipitation and ultrafiltration. Desalination Water Treat 9:1–8. https://doi.org/10.5004/dwt.2009.745
Chéry Leal MJ, do Amaral PAP, Nagel-Hassemer ME et al (2015) Aquatic humic substances, iron, and manganese removal by ultrafiltration and nanofiltration membranes combined with coagulation–flocculation–sedimentation. Desalination Water Treat 55:1662–1671. https://doi.org/10.1080/19443994.2015.1012337
Hwang KJ, Liao CY, Tung KL (2007) Analysis of particle fouling during microfiltration by use of blocking models. J Membr Sci 287(2):287–293. https://doi.org/10.1016/j.memsci.2006.11.004
Lee EK, Chen V, Fane AG (2008) Natural organic matter (NOM) fouling in low pressure membrane filtration––effect of membranes and operation modes. Desalination 218(1–3):257–270. https://doi.org/10.1016/j.desal.2007.02.021
Ahmed N, Mir FQ (2021) Chromium (VI) removal using micellar enhanced microfiltration (MEMF) from an aqueous solution: fouling analysis and use of ANN for predicting permeate flux. J Water Process Eng 44:102438. https://doi.org/10.1016/j.jwpe.2021.102438
Ahmed N, Mir FQ (2021) Fabrication of a cost effective ceramic microfiltration membrane by utilizing local Kashmir clay. Trans Indian Ceram Society 80:41–46. https://doi.org/10.1080/0371750X.2020.1864663
Khemakhem S, Larbot A, ben Amar R (2009) New ceramic microfiltration membranes from Tunisian natural materials: application for the cuttlefish effluents treatment. Ceram Int 35:55–61. https://doi.org/10.1016/j.ceramint.2007.09.117
Jana S, Purkait MK, Mohanty K (2010) Preparation and characterization of low-cost ceramic microfiltration membranes for the removal of chromate from aqueous solutions. Appl Clay Sci 47:317–324. https://doi.org/10.1016/j.clay.2009.11.036
Emani S, Uppaluri R, Purkait MK (2013) Preparation and characterization of low cost ceramic membranes for mosambi juice clarification. Desalination 317:32–40. https://doi.org/10.1016/j.desal.2013.02.024
Vasanth D, Pugazhenthi G, Uppaluri R (2011) Fabrication and properties of low cost ceramic microfiltration membranes for separation of oil and bacteria from its solution. J Membr Sci 379:154–163. https://doi.org/10.1016/j.memsci.2011.05.050
Varol B, Uzal N (2015) Arsenic removal from aqueous solutions by ultrafiltration assisted with polyacrylamide: an application of response surface methodology. Desalin Water Treat 56:736–743. https://doi.org/10.1080/19443994.2014.937765
Myers RH, Montgomery DC (2002) Response surface methodology: process and product optimization using designed experiments, 2nd edn. Wiley
Montgomery DC (2001) Design and analysis of experiments, 5th edn. Wiley, New York
Chakraborty S, Dasgupta J, Farooq U et al (2014) Experimental analysis, modeling and optimization of chromium (VI) removal from aqueous solutions by polymer-enhanced ultrafiltration. J Membr Sci 456:139–154. https://doi.org/10.1016/j.memsci.2014.01.016
Suresh K, Pugazhenthi G, Uppaluri R (2016) Fly ash based ceramic microfiltration membranes for oil-water emulsion treatment: parametric optimization using response surface methodology. J Water Process Eng 13:27–43. https://doi.org/10.1016/j.jwpe.2016.07.008
Alipanahpour Dil E, Ghaedi M, Ghezelbash GR et al (2017) Highly efficient simultaneous biosorption of Hg 2+, Pb 2+ and Cu 2+ by Live yeast Yarrowia lipolytica 70562 following response surface methodology optimization: Kinetic and isotherm study. J Ind and Eng Chem 48:162–172. https://doi.org/10.1016/j.jiec.2016.12.035
Belgada A, Charik FZ, Achiou B et al (2021) Optimization of phosphate/kaolinite microfiltration membrane using Box–Behnken design for treatment of industrial wastewater. J Environ Chem Eng 9:104972. https://doi.org/10.1016/j.jece.2020.104972
Rai P, Pandey A, Pandey A (2019) Optimization of sugar release from banana peel powder waste (BPPW) using Box-Behnken design (BBD): BPPW to biohydrogen conversion. Int J Hydrog s 44:25505–25513. https://doi.org/10.1016/j.ijhydene.2019.07.168
Uddin MK, Nasar A (2020) Walnut shell powder as a low-cost adsorbent for methylene blue dye: isotherm, kinetics, thermodynamic, desorption and response surface methodology examinations. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-64745-3
Nourbakhsh H, Emam-Djomeh Z, Omid M et al (2014) Prediction of red plum juice permeate flux during membrane processing with ANN optimized using RSM. Comput Electron Agric 102:1–9. https://doi.org/10.1016/j.compag.2013.12.017
Idris A, Kormin F, Noordin M (2006) Application of response surface methodology in describing the performance of thin film composite membrane. Sep Purif Technol 49:271–280. https://doi.org/10.1016/j.seppur.2005.10.010
Xiarchos I, Jaworska A, Zakrzewska-Trznadel G (2008) Response surface methodology for the modelling of copper removal from aqueous solutions using micellar-enhanced ultrafiltration. J Membr Sci 321:222–231. https://doi.org/10.1016/j.memsci.2008.04.065
Khayet M, Seman MNA, Hilal N (2010) Response surface modeling and optimization of composite nanofiltration modified membranes. J Membr Sci 349:113–122. https://doi.org/10.1016/j.memsci.2009.11.031
Gao XK, Low TS, Liu ZJ, Chen SX (2002) Robust design for torque optimization using response surface methodology. IEEE Trans Magn 38:1141–1144. https://doi.org/10.1109/20.996292
Zhao H, Tonkyn RG, Barlow SE et al (2006) Fractional factorial study of HCN removal over a 0.5% Pt/Al2O3 catalyst: effects of temperature, gas flow rate, and reactant partial pressure. Ind Eng Chem Res 45:934–939. https://doi.org/10.1021/ie048777e
Palamakula A, Nutan MTH, Khan MA (2004) Response surface methodology for optimization and characterization of limonene-based coenzyme Q10 self-nanoemulsified capsule dosage form. AAPS PharmSciTech 5:114–121. https://doi.org/10.1208/pt050466
Yetilmezsoy K, Demirel S, Vanderbei RJ (2009) Response surface modeling of Pb(II) removal from aqueous solution by Pistacia vera L.: Box-Behnken experimental design. J Hazard Mater 171:551–562. https://doi.org/10.1016/j.jhazmat.2009.06.035
Workneh S, Shukla A (2008) Synthesis of sodalite octahydrate zeolite-clay composite membrane and its use in separation of SDS. J Membr Sci 309:189–195. https://doi.org/10.1016/j.memsci.2007.10.033
Almandoz MC, Marchese J, Prádanos P et al (2004) Preparation and characterization of non-supported microfiltration membranes from aluminosilicates. J Membr Sci 241:95–103. https://doi.org/10.1016/j.memsci.2004.03.045
APHA (1992) Standard methods for the examination of water and wastewater, 18th ed. Washington, DC.
Acknowledgements
The authors acknowledge the Central Research Facility (CRF), NIT Srinagar for help with the XRD and FESEM analysis of prepared samples.
Funding
This work was funded by a grant (No. NIT/TEQIP/19/1361) from TEQIP III, NIT Srinagar under the budget head "Research and Development".
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Andrea de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, N., Mir, F.Q. Box–Behnken design for optimization of iron removal by hybrid oxidation–microfiltration process using ceramic membrane. J Mater Sci 57, 15224–15238 (2022). https://doi.org/10.1007/s10853-022-07567-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07567-0