Abstract
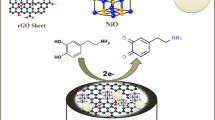
In this work, rGO-ZnO (reduced graphene oxide–zinc oxide) nanocomposite was prepared and used for modification of GC (glassy carbon) surface in order to obtain an electrochemical sensor for tetracycline determination in water and urine samples by voltammetric technique. The characterization of synthesized nanocomposite was accomplished by utilizing SEM, EDS, AFM and FTIR analyses. The electrochemical behaviour of tetracycline on the modified GCE was studied by cyclic voltammetry and results revealed that modification enhanced the electro-oxidation of tetracycline with increased current intensity. Tetracycline provided a well-defined oxidation peak at around + 1.05 V vs. Ag/AgCl (3.5 mol/L KCl) in Britton–Robinson buffer (BRB) at pH 8.0. Then, square-wave voltammetry (SWV) was applied for analytical purposes. The influence of the supporting electrolyte (type, concentration and pH) and SWV parameters on the peak current was investigated in order to optimize the experimental and instrumental conditions for quantitative analysis. Under the optimal conditions, the prepared sensor exhibits a wide linear range (4–400 µmol/L) with a low limit of detection (0.38 µmol/L) and good reproducibility of analysis (RSD < 3.40%). In addition, the sensor showed high selectivity towards tetracycline analysis in comparison to interferences often present in real samples. The practical analytical usefulness of the presented sensing platform was successfully demonstrated in the determination of tetracycline in water and human urine with good recoveries.
Graphical abstract











Similar content being viewed by others
References
Chopra I, Roberts M (2001) Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol Mol Biol Rev 65:232–260. https://doi.org/10.1128/mmbr.65.2.232-260.2001
Roberts MC (2003) Tetracycline therapy: Update. Clin Infect Dis 36:462–467
Nelson ML (2001) The chemistry and cellular biology of the tetracyclines. In: Nelson M, Greenwald W, Hillen RA (eds) Tetracyclines in Biology, Chemistry and Medicine. Birkhäuser, Basel, pp 3–63
Granados-Chinchilla F, Rodríguez C (2017) Tetracyclines in Food and Feedingstuffs: From Regulation to Analytical Methods, Bacterial Resistance, and Environmental and Health Implications. J Anal Methods Chem Article ID. https://doi.org/10.1155/2017/1315497
Baietto L, Corcione S, Pacini G, Perri GD, D’Avolio A, De Rosa FG (2014) A 30-years Review on Pharmacokinetics of Antibiotics: Is the Right Time for Pharmacogenetics? Curr Drug Metab 15:581–598
Bound JP, Voulvoulis N (2004) Pharmaceuticals in the aquatic environment - A comparison of risk assessment strategies. Chemosphere 56:1143–1155. https://doi.org/10.1016/j.chemosphere.2004.05.010
Aalipour F, Mirlohi M, Jalali M, Azadbakht L (2015) Dietary exposure to tetracycline residues through milk consumption in Iran. J Environ Heal Sci Eng 13:1–7. https://doi.org/10.1186/s40201-015-0235-6
Borghi AA, Palma MSA (2014) Tetracycline: Production, waste treatment and environmental impact assessment. Brazilian J Pharm Sci 50:25–40. https://doi.org/10.1590/S1984-82502011000100003
Zhao Y, Geng J, Wang X et al (2011) Tetracycline adsorption on kaolinite: PH, metal cations and humic acid effects. Ecotoxicology 20:1141–1147. https://doi.org/10.1007/s10646-011-0665-6
Pérez-Rodríguez M, Pellerano RG, Pezza L, Pezza HR (2018) An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 182:1–21. https://doi.org/10.1016/j.talanta.2018.01.058
Al-Afy N, Sereshti H, Hijazi A, Rashidi Nodeh H (2018) Determination of three tetracyclines in bovine milk using magnetic solid phase extraction in tandem with dispersive liquid-liquid microextraction coupled with HPLC. J Chromatogr B Anal Technol Biomed Life Sci 1092:480–488. https://doi.org/10.1016/j.jchromb.2018.06.049
Cherkashina K, Vakh C, Lebedinets S et al (2018) An automated salting-out assisted liquid-liquid microextraction approach using 1-octylamine: On-line separation of tetracycline in urine samples followed by HPLC-UV determination. Talanta 184:122–127. https://doi.org/10.1016/j.talanta.2018.02.112
Phiroonsoontorn N, Sansuk S, Santaladchaiyakit Y, Srijaranai S (2017) The use of dissolvable layered double hydroxide components in an in situ solid-phase extraction for chromatographic determination of tetracyclines in water and milk samples. J Chromatogr A 1519:38–44. https://doi.org/10.1016/j.chroma.2017.09.005
Chico J, Meca S, Companyó R et al (2008) Restricted access materials for sample clean-up in the analysis of trace levels of tetracyclines by liquid chromatography. Application to food and environmental analysis. J Chromatogr A 1181:1–8. https://doi.org/10.1016/j.chroma.2007.12.033
Koesukwiwat U, Jayanta S, Leepipatpiboon N (2007) Validation of a liquid chromatography-mass spectrometry multi-residue method for the simultaneous determination of sulfonamides, tetracyclines, and pyrimethamine in milk. J Chromatogr A 1140:147–156. https://doi.org/10.1016/j.chroma.2006.11.099
Ibarra IS, Rodriguez JA, Miranda JM et al (2011) Magnetic solid phase extraction based on phenyl silica adsorbent for the determination of tetracyclines in milk samples by capillary electrophoresis. J Chromatogr A 1218:2196–2202. https://doi.org/10.1016/j.chroma.2011.02.046
Gao F, Zhao GX, Zhang HC et al (2013) Production of monoclonal antibody against doxycycline for immunoassay of seven tetracyclines in bovine muscle and milk. J Environ Sci Heal Part B 48:92–100. https://doi.org/10.1080/03601234.2013.726856
Sattayasamitsathit S, Thavarungkul P, Kanatharana P (2007) Bismuth film electrode for analysis of tetracycline in flow injection system. Electroanalysis 19:502–505. https://doi.org/10.1002/elan.200603726
Calixto CMF, Cavalheiro ÉTG (2015) Determination of Tetracyclines in Bovine and Human Urine using a Graphite-Polyurethane Composite Electrode. Anal Lett 48:1454–1464
Calixto CMF, Cervini P, Cavalheiro ÉTG (2012) Determination of tetracycline in environmental water samples at a graphite-polyurethane composite electrode. J Braz Chem Soc 23:938–943. https://doi.org/10.1590/S0103-50532012000500020
Rajab Dizavandi Z, Aliakbar A, Sheykhan M (2017) A novel Pb-poly aminophenol glassy carbon electrode for determination of tetracycline by adsorptive differential pulse cathodic stripping voltammetry. Electrochim Acta 227:345–356. https://doi.org/10.1016/j.electacta.2016.12.167
Guo G, Zhao F, Xiao F, Zeng B (2009) Voltammetric determination of tetracycline by using multi-wall carbon nanotube - ionic liquid film coated glassy carbon electrode. Int J Electrochem Sci 4:1365–1372
Kesavan S, Kumar DR, Lee YR, Shim JJ (2017) Determination of tetracycline in the presence of major interference in human urine samples using polymelamine/electrochemically reduced graphene oxide modified electrode. Sensors Actuators, B Chem 241:455–465. https://doi.org/10.1016/j.snb.2016.10.091
Kushikawa RT, Silva MR, Angelo ACD, Teixeira MFS (2016) Construction of an electrochemical sensing platform based on platinum nanoparticles supported on carbon for tetracycline determination. Sensors Actuators, B Chem 228:207–213. https://doi.org/10.1016/j.snb.2016.01.009
Wong A, Scontri M, Materon EM et al (2015) Development and application of an electrochemical sensor modified with multi-walled carbon nanotubes and graphene oxide for the sensitive and selective detection of tetracycline. J Electroanal Chem 757:250–257. https://doi.org/10.1016/j.jelechem.2015.10.001
Allahverdiyeva S, Yardım Y, Şentürk Z (2021) Electrooxidation of tetracycline antibiotic demeclocycline at unmodified boron-doped diamond electrode and its enhancement determination in surfactant-containing media. Talanta. https://doi.org/10.1016/j.talanta.2020.121695
Taghioskoui M (2009) Trends in graphene research Mater Today 12:34–37. https://doi.org/10.1016/S1369-7021(09)70274-3
Tarcan R, Todor-Boer O, Petrovai I et al (2020) Reduced graphene oxide today. J Mater Chem C 8:1198–1224. https://doi.org/10.1039/c9tc04916a
Rowley-Neale SJ, Randviir EP, Abo Dena AS, Banks CE (2018) An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl Mater Today 10:218–226. https://doi.org/10.1016/j.apmt.2017.11.010
Liu H, Zhang L, Guo Y et al (2013) Reduction of graphene oxide to highly conductive graphene by Lawesson’s reagent and its electrical applications. J Mater Chem C 1:3104–3109. https://doi.org/10.1039/c3tc00067b
Smith AT, LaChance AM, Zeng S et al (2019) Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater Sci 1:31–47. https://doi.org/10.1016/j.nanoms.2019.02.004
Govindasamy M, Wang SF, Pan WC et al (2019) Facile sonochemical synthesis of perovskite-type SrTiO3 nanocubes with reduced graphene oxide nanocatalyst for an enhanced electrochemical detection of α-amino acid (tryptophan). Ultrason Sonochem 56:193–199. https://doi.org/10.1016/j.ultsonch.2019.04.004
Govindasamy M, Wang SF, Subramanian B et al (2019) A novel electrochemical sensor for determination of DNA damage biomarker (8-hydroxy-2′-deoxyguanosine) in urine using sonochemically derived graphene oxide sheets covered zinc oxide flower modified electrode. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2019.104622
Jandaghi N, Jahani S, Kazemipour M et al (2019) One-pot synthesis of cerium doped 3D ZnO nano-flowers modified on glassy carbon electrode as portable electro-chemical sensing platform for sensitive detection of methotrexate as an anticancer drug. Synth Met 256:116119. https://doi.org/10.1016/j.synthmet.2019.116119
Ezhil Vilian AT, Kang SM, Yeong OhS et al (2020) A simple strategy for the synthesis of flower-like textures of Au-ZnO anchored carbon nanocomposite towards the high-performance electrochemical sensing of sunset yellow. Food Chem 323:126848. https://doi.org/10.1016/j.foodchem.2020.126848
Jain R, Thakur A, Kumar P, Pooja D (2018) Au/ZnO nanocomposites decorated ITO electrodes for voltammetric sensing of selenium in water. Electrochim Acta 290:291–302. https://doi.org/10.1016/j.electacta.2018.09.061
Marlinda AR, Pandikumar A, Yusoff N et al (2015) Electrochemical sensing of nitrite using a glassy carbon electrode modified with reduced functionalized graphene oxide decorated with flower-like zinc oxide. Microchim Acta 182:1113–1122. https://doi.org/10.1007/s00604-014-1436-x
Hummers WS, Offeman RE (1958) Preparation of Graphitic Oxide. J Am Chem Soc 80:1339. https://doi.org/10.1021/ja01539a017
Debbarma M, Das S, Saha M (2013) Effect of reducing agents on the structure of zinc oxide under microwave irradiation. Adv Manuf 1:183–186. https://doi.org/10.1007/s40436-013-0020-7
ICDD (2020). PDF-4+ 2021. International Centre for Diffraction Data, Newtown Square, PA, USA.
Degen T, Sadki M, Bron E et al (2014) The high score suite. Powder Diffr 29:S13–S18. https://doi.org/10.1017/S0885715614000840
Scherrer P (1918) Estimation of the Size and Internal Structure of Colloidal Particles by Means of Röntgen. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen 2:96–100
Periasamy M, Thirumalaikumar M (2000) Methods of enhancement of reactivity and selectivity of sodium borohydride for applications in organic synthesis. J Organomet Chem 609:137–151. https://doi.org/10.1016/S0022-328X(00)00210-2
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun 143:47–57. https://doi.org/10.1016/j.ssc.2007.03.052
Damen TC, Porto SPS, Tell B (1966) Raman Effect in ZnO. Phys Rev 142:570–573
Calleja JM, Cardona M (1977) Resonant Raman scattering in ZnO. Phys Rev B 16:3753–3761. https://doi.org/10.1103/PhysRevB.16.3753
Shin H-J, Kim KK, Benayad A, Yoon S-M, Park HK, Jung I-S, Jin MH, Jeong H-K, Kim JM, Jae-Young Choi YHL (2009) Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv Funct Mater 19:1987–1992
Liu G, Wang L, Wang B et al (2015) A reduced graphene oxide modified metallic cobalt composite with superior electrochemical performance for supercapacitors. RSC Adv 5:63553–63560. https://doi.org/10.1039/c5ra09748g
Gomez-Alvarez MA, Morales C, Méndez J et al (2020) A Comparative Study of the ZnO Growth on Graphene and Graphene Oxide: The Role of the Initial Oxidation State of Carbon. The Role of the Initial Oxidation State of Carbon C 6(2):41. https://doi.org/10.3390/c6020041
Lesiak B, Kövér L, Tóth J et al (2018) C sp2/sp3 hybridisations in carbon nanomaterials – XPS and (X)AES study. Appl Surf Sci 452:223–231. https://doi.org/10.1016/j.apsusc.2018.04.269
Tay YY, Li S (2006) Size dependence of Zn 2p 3/2 binding energy in nanocrystalline ZnO. Appl Phys 88(17):173118. https://doi.org/10.1063/1.2198821
Moretti G (1998) Auger parameter and Wagner plot in the characterization of chemical states by X-ray photoelectron spectroscopy: A review. J Electron Spectrosc Relat Phenom 95(2–3):95–144. https://doi.org/10.1016/S0368-2048(98)00249-7
Sutar DS, Kushwaha N, Appani SK, Major SS (2020) Energy level alignment of graphene oxide and its derivatives with ZnO. J Electron Spectrosc Relat Phenom 23:146953. https://doi.org/10.1016/j.elspec.2020.146953
Fortgang P, Tite T, Barnier V et al (2016) Robust Electrografting on Self-Organized 3D Graphene Electrodes. ACS Appl Mater Interfaces 8:1424–1433. https://doi.org/10.1021/acsami.5b10647
Stojanović ZS, Đurović AD, Ashrafi AM et al (2020) Highly sensitive simultaneous electrochemical determination of reduced and oxidized glutathione in urine samples using antimony trioxide modified carbon paste electrode. Sensors Actuators, B Chem. https://doi.org/10.1016/j.snb.2020.128141
Wang H, Robinson JT, Diankov G, Dai H (2010) Nanocrystal growth on graphene with various degrees of oxidation. J Am Chem Soc 132:3270–3271. https://doi.org/10.1021/ja100329d
Huang HP, Zhu JJ (2011) Preparation of novel carbon-based nanomaterial of graphene and its applications electrochemistry. Fenxi Huaxue/ Chinese J Anal Chem 39:963–971. https://doi.org/10.1016/S1872-2040(10)60450-1
Vega D, Agüí L, González-Cortés A et al (2007) Voltammetry and amperometric detection of tetracyclines at multi-wall carbon nanotube modified electrodes. Anal Bioanal Chem 389:951–958. https://doi.org/10.1007/s00216-007-1505-7
European Medicines Agency (1995) VALIDATION OF ANALYTICAL PROCEDURES: TEXT AND METHODOLOGY Q2(R1). Eur Med Agency 1–15
Acknowledgements
This work was supported by European Regional Development Fund "Multidisciplinary research to increase application potential of nanomaterials in agricultural practice" (No. CZ.02.1.01/0.0/0.0/16_025/0007314) and by the Ministry of Education, Youth and Sports of the Czech Republic under the Central European Institute of Technology (CEITEC) 2020 project (LQ1601). We acknowledged the Nanobiotechnology core facility supported by the Ministry of Education, Youth and Sports of the Czech Republic (LM2018127). Authors from the Faculty of Technology, University of Novi Sad acknowledge support from the Ministry of Education, Science and Technological development, Republic of Serbia (Project No. 451–03-68/2022–14/200134). The authors also acknowledge the assistance provided by the Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project No. LM2018124.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Joshua Tong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Đurović, A., Stojanović, Z., Bytešníková, Z. et al. Reduced graphene oxide/ZnO nanocomposite modified electrode for the detection of tetracycline. J Mater Sci 57, 5533–5551 (2022). https://doi.org/10.1007/s10853-022-06926-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-06926-1