Abstract
Magnetron sputtering is one of the most commonly used deposition techniques, which has received considerable attention in industrial applications. In particular, owing to its compatibility with conventional fabrication processes, it can produce and fabricate high-quality dense thin films of a wide range of materials. In the present study, nitrogen (N) was combined with pure vanadium in order to form binary nitride to improve its mechanical and tribological performance. To evaluate the influence of nitrogen on the structure of the as-deposited vanadium nitride (VN) coatings, the following techniques were used: XPS, XRD, SEM, AFM and optical profilometry. The residual stresses were determined by the curvature method using Stoney’s formula. The hardness and Young’s modulus were obtained by nanoindentation measurements. The friction behavior and wear characteristics of the films were evaluated by using a ball-on-disk tribometer. The obtained results showed that the N/V ratio increased with increasing the N2 flow rate while the deposition rate decreased. The preferred orientation was changed from (200) to (111) as the N2 flow rate increased with the presence of V–N and V–O binding energies as confirmed by XPS analysis. The nitrogen addition resulted in a columnar morphology and a fine structure with fine surface roughness. The VN thin film containing 49.5 at.% of nitrogen showed the best performance: highest mechanical properties (hardness = 25 GPa), lowest friction coefficient (μ = 0.37) and lowest wear rate (Ws = 2.72 × 10−5 mm3N−1 m−1). A good correlation between the film microstructure, crystallite size, residual stress and mechanical and tribological properties was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hard phases corresponding to transition metal nitrides are capable of retaining their mechanical properties such as strength and hardness under extreme conditions, because of which, they have been receiving considerable attention in the recent past [1,2,3]. Vanadium nitride (VN) is one such transition metal nitride, which has found its way in a wide variety of applications in many industrial fields, owing to their excellent properties such as high hardness and wear resistance, good electrical and thermal conductivity[4, 5], good catalytic activity, and excellent thermal stability [6,7,8].,. VN particularly in the form of thin film is extensively used in industrial devices requiring high performance, corrosion resistance and improved abrasion resistance in microelectronics, superconductors and sometimes as decorative coatings. However, they are widely used as hard coatings for cutting tools to improve their service life and performance [9,10,11,12,13,14].
The fcc-VN compound has six-coordinate nitrogen (N) and eight-coordinate N in its structure and the crystal structure of high-pressure V2N phase has a hexagonal structure in which N atoms occupy the octahedral interstitial sites [10]. Hofer et al. [11] reported a rapid thermal processing of vanadium layers in pure nitrogen at elevated temperatures, and their electron diffraction patterns revealed different phases: fcc-VN structure was identified around 1100 °C and the hexagonal structure V2N was found at 900 °C. In an another study, XRD measurements of VN samples showed that the films deposited at 150 °C at lower nitrogen gas flow, exhibit significantly higher hardness as compared to that of the transition metal nitrides phase with NaCl structure [12].
It is well-known that the deposition parameters such as the substrate temperature, deposition time, substrate bias and nitrogen flow rate influence the structural, mechanical and tribological performance of the produced VN thin films [2]. Chu et al. [15] showed that the film morphology, phase transformation and properties of sputtered VN thin films were related to the film microstructural changes, which are controlled by the deposition parameters such as nitrogen partial pressure, substrate bias and target power. A mixture of crystalline hcp-V2N and fcc-VN phases was obtained at about 50 and 60 at.% of vanadium (V). Maximum hardness of 29 GPa was found for the V2Nx phase produced at a nitrogen pressure of 1.33 Pa. Suszko et al. [16] reported that the preferred orientation was changed with nitrogen pressure from (311) through (111) to (200) planes parallel to the substrate surface. The preferential (200) orientation was observed at the highest nitrogen partial pressure (Ar/N2 = 0.35/1.2 Pa).
Caicedo et al. [17]. produced VN films from a metallic V target sputtered in a gaseous atmosphere of (N2 + Ar) mixture at different D.C. negative bias voltages. Nanoindentation measurements showed that increasing the bias voltage from 0to − 150 V enhanced the film hardness and elastic modulus up to 20 GPa (H) and 221 GPa (E), respectively. A preferential orientation of fcc-VN (200) plane was observed. Moreover, the VN coating deposited at the highest D.C. bias voltage (− 150 V) presented a greater corrosion rate than that obtained for the film deposited without substrate polarization. Qiu et al. [18]. reported that increasing the nitrogen content influenced the density of coatings, their maximum hardness (22.9 GPa) and residual stresses (–1.62 GPa) generated during the deposition process.
Ina previous work, the effect of film thickness and (Ar-N2) plasma gas on the structure and performance of VN coatings deposited by R.F. magnetron sputtering was studied. At 10%of N2, the presence of the hcp-V2N phase was observed. Then, the structure changed from single hcp-V2N to combination of hcp-V2N and fcc-VN when the nitrogen increased up to 20%. The thicker films containing a high amount of nitrogen were slightly denser as compared to the thinner ones presenting rough surface and a columnar structure. The highest hardness of 26.2 GPa was obtained for the VN film deposited under 20% of N2, which is correlated with its dense structure and stoichiometric composition. Furthermore, a lower friction coefficient of about 0.4 was found for the thickest film of 2.5 µm [21].
However, limited reports are available investigating the effect of chemical compositions and bonding structure on the tribo-mechanical properties of V–N [17,18,19,20,21]. Moreover, most of the previous studies have reported only the hardness of VN (13 GPa) phase [19], while the mechanical properties of other V–N compounds are still rarely reported due to the difficulty of preparing the samples with different stoichiometries. Therefore, a comprehensive investigation on the influence of the deposition parameters on the structure as well as tribo-mechanical properties is required, in order to suitably adjust the properties of V–N films. Hence, the current study is focused on the microstructure evaluation and relates the chemical compositions and bonding structure to mechanical properties, adhesion and tribological performance of VN films. By controlling the N2 flow rate, a series of VN thin films with various compositions and morphologies were prepared. The effect of nitrogen content on microstructure, mechanical and tribological performance of VN thin films is explored in detail. In contrast to the reports in the literature, results of this work showed a significant dependence of film behavior on its microstructure and confirmed a substantial enhancement in friction and wear characteristics of XC100 steel coated by VN thin films that could be used in a wide range of industrial applications.
Experimental details
Substrate preparation
XC100 steel substrates (Ø 15 mm × 3 mm) were grinded by abrasive papers and polished with a diamond suspension to obtain a RMS roughness of about 30 nm. Steel substrates and Si (100) wafers (10 mm × 10 mm × 480 μm) were ultrasonically cleaned with acetone and ethanol for 10 min, rinsed with deionized water and dried with compressed air. The as-deposited films on the XC100 substrates were used to investigate the microstructure, mechanical and tribological properties, while coatings on Si (100) wafers were employed to observe the surface topography, cross-sectional morphologies as well as to measure the residual stress.
Film deposition
VN thin films were deposited by reactive R.F. magnetron sputtering (NORDIKO type 3500, 13.56 MHz) from circular V target (99.9% purity, Ø 10.6 cm). Detailed description of sputtering process is presented in our earlier study [21]. Target to substrate distance was kept constant at 80 mm for all depositions. The deposition temperature in the process chamber was about 150 °C. Prior to deposition, the chamber was evacuated to a low pressure of 6 × 10−4 Pa. The total sputtering (Ar + N2) gas flow rate was 100 sccm, which maintained the working pressure at 0.4 Pa. In order to remove the surface oxides and to etch the surface, the substrates were initially cleaned using an argon flow rate of 80 sccm, a pressure of 0.4 Pa and a bias voltage of -200 V. Vanadium target was cleaned in Ar using a voltage of 700 V for 10 min to remove the surface oxides and contaminations.
In order to enhance the VN film-to-substrate adhesion, a pure vanadium interlayer of ~ 60 nm was deposited on the substrates prior to the actual film deposition by applying a power of 250 W to the vanadium target for 5 min under a pure argon gas atmosphere with a flow rate of 50 sccm.
VN thin films were deposited by simultaneously injecting Ar and N2 into the chamber for 120 min and the sputtering RF power (bias voltage) for V target was kept at 550 W (-900 V). Five different configurations of gas mixtures were used with varying nitrogen flow rates of 5, 10, 15, 20 and 25 sccm.
Film characterization techniques
VN thin films were deposited on the Si (100) wafers for physicochemical characterizations, determination of residual stresses, and observation of the surface morphology and cross section of the films by SEM and topography by AFM. However, the VN thin films deposited on the XC100 steel substrates were used for the analysis by X-ray diffraction, nanoindentation measurements and for tribological tests.
The crystalline structure phases of the as-deposited films were identified by X-ray diffraction (XRD, type Bruker D8t) with Co-Kα radiation (λ = 0.178 nm) at 40 kV and 35 mA. The film spectra were obtained using (θ–2θ) angle diffraction by varying 2θ between 25° and 80° with a 0.02° scan step. In order to determine the preferred orientation degree, texture coefficient (Tc) was calculated using the following formula [22]:
where I(hkl) film is the normalized integral intensity of the (hkl) diffraction peak, I(hkl)bulk is the integral intensity of the bulk material peak, N is the number of consider peaks.
The ratio of (hkl) orientation (%) was calculated using the following equation:
where I(hkl)film is the normalized intensity of the (hkl) diffraction peak, which is equal to the top the (hkl) peak on the XRD pattern; I(hkl)bulk is the integral intensity of the bulk material peak after correction.
The crystallite size (D) of the VN thin films was calculated using Scherrer’s formula [23]:
where 0.9 is a dimensionless shape factor, λthe wavelength of the CoKα incidence, β the width at half-maximum of the diffraction peak and θ the Bragg’s angle of VN phase.
The lattice strains of the (111) VN plane were calculated using the following equation:
where ɛ is the lattice strain in the a axis direction,\(\Delta {\mathbf{a}} = \left( {{\mathbf{a}}_{{{\mathbf{coating}}}} - {\mathbf{a}}_{{{\mathbf{bulk}}}} } \right)\), acoating and abulk present the cubic VN phase.
The ratio of (hkl) orientation (%) was calculated using the following equation:
where I(hkl)film is the normalized intensity of the (hkl) diffraction peak, which is equal to the intensity of (hkl) peak on the XRD pattern; I(hkl)bulk is the integral intensity of the bulk material peak after correction.
The binding energy of the VN thin films was determined by X-ray Photoelectron Spectroscopy (XPS, Riber SIA 100 spectrometer) with a non-monochromatic X-ray source (AlKα line of 1486.6 eV), an applied power of 300 W and a vacuum pressure of 4 × 10–8 Pa. The calibration was done using the line of C1s of about 284.5 eV and a full width at half-maximum (FWHM) of 1.1 eV, which was absorbed on the sample surface [24]. In the XPS analysis, C1s has made a spectacular referencing due to the fact that thin C contamination layers, in the form of so-called adventitious carbon are present on all surfaces that have been previously exposed to the atmosphere [4, 24]. Hence, calibrating the XPS scale to the C1s peak set at 284.5 eV is justified and may lead to physical results, like a zero density of states at the Fermi level especially for conducting samples such as metals, where charging effects can definitely be excluded [24].The V2p, O1 and N1s spectra were obtained by Casa XPS software analysis [25].
Cross-sectional images of the films deposited on Si (100) wafers were observed by a Scanning Electron Microscope (SEM, JEOL JSM-6400F). The elemental analysis of the films was determined by Energy Dispersive Spectroscopy (EDS, Oxford INCA x-act). The surface roughness and three-dimensional topographies of the films were characterized by an Atomic Force Microscopy (AFM, Type 100, APE research) in a contact mode by applying a constant force of 0.032 N/m and scanning an area of 3 × 3 μm2.
The residual stresses of the VN thin films were calculated using the following Stoney’s equation [26]:
where Es is the substrate Young’s modulus, νs the substrate Poisson’s ratio, ts the substrate thickness, tf the film thickness, R0 the curvature radius of sample before deposition and R the curvature radius of the coated sample measured using an optical profilometer.
The mechanical properties of the films (hardness and Young’s modulus) were obtained using a nanoindenter equipped with a Berkovich diamond tip (Nanoindenter MTS-XP) by applying a load of 50 mN. The mechanical properties were calculated using the Oliver-Pharr method [27]. The hardness values were determined from the average of 5 indentations. The maximum indenter penetration was limited to 10% of the total film thickness in order to avoid the influence of substrate and surface roughness. The friction and wear performances of the as-deposited films were tested by a ball-on-disk tribometer using a 100Cr6 ball as a counterpart (Φ = 6 mm). The tests were carried out applying a normal load of 2 N with a sliding speed of 0.05 m/s for 20 min. The friction coefficient curves were recorded by software associated with the data acquisition. The worn wear tracks, depth and wear rates were determined by means of an optical 3D profilometer (VEECO, Wyko-NT 1100) [2]. The worn surfaces were analyzed by using SEM and EDS.
Results and discussion
Structural analysis
Table 1 presents the elemental composition of films deposited under different nitrogen flow rates. It is clearly observed that nitrogen content in the VN thin films significantly increased from 15.4 to 51.3 at.% with increasing N2 flow rate from 5 to 25 sccm. N/V ratio increased from 0.18 to 1.10 and the deposition rate gradually decreased from 10.8 nm/min to 7.5 nm/min (Table 1). Since the deposition rate is strongly dependent on the number of N and V atoms arriving upon the substrate surface, low deposition rate was due to the reduction of V concentration in the VN thin film that is confirmed by the increasing of N/V ratio [28]. This is attributed to the target contamination, which is consistent with the results reported by Huang et al. [22] showing a similar decrease in the Ti content in TiN thin films as a function of increasing nitrogen flow rate. The presence of oxygen in a low percentage (2–2.8 at.%) is probably due to the moderate vacuum and the presence of residual oxygen in the deposition chamber [21].
The XPS core-level spectra in the V2p, N1s and O1s regions, for the VN thin films deposited under 10 and 20 sccm of nitrogen, are shown in Fig. 1. XPS spectra indicated that there are not only V-V and N-V binding energies but also V–O. At 10 sccm, the V2p3/2 has two peaks (Fig. 1a). The first peak is centered at the VN binding energy of 514.62 eV [2] while the second peak is centered at 514.82 eV corresponding to the V2N binding energy [29]. The V2p1/2 peak is centered at 522.42 eV corresponding to VN and V2N phases, respectively [2, 29]. For the N1s (Fig. 1b), the dominant binding energy was around 396.25 eV corresponding to V–N bonds of VN and V2N [30].
The difference in the binding energies (∆BE) between the N1s ± Vp levels was also calculated. The ∆BE values between the N1s ± V2p levels were found to be decreasing with increasing nitrogen content according to the position of their V2p and N 1 s levels. The chemical shift of the core levels can be attributed to the change in the charge distribution providing an indication of the degree of ionicity and covalency of the bonding [31].
The O1s spectrum becomes wider and less intense, with one peak at about 530.49 eV consistent with V–O binding energy (Fig. 1c) [33]. At 20 sccm, the V2p3/2 peak includes two components, corresponding to nitride VN (513.32 eV) and oxide V2O5(516.03 eV), respectively (Fig. 1d) [32]. Peak corresponding to V2N phase disappeared at 20 sccm. The strong N1s peak is centered at 396.15 eV corresponding to V–N and V–O-N binding energies (Fig. 1e) [33, 34]. This indicates that the V–N bond is strongly formed in the VN thin films after the incorporation of nitrogen atoms and confirms transformation of V2N into VN and coexistence of vanadium nitride (VN) and oxide phases in films deposited at 20 sccm of N2 as shown later in XRD analysis. N1s peak has a higher intensity for the film deposited at 20 sccm of N2 than that obtained at 10 sccm. This difference means, probably, that nitrogen is rather linked to vanadium during the film deposition. Generally, the V–O binding energy observed in the XPS spectra is due to the moderate vacuum and residual oxygen in deposition chamber. Therefore, the binding energy of O1s core level, taken at 530.50 eV is corresponding toV-O (Fig. 1f) [33].
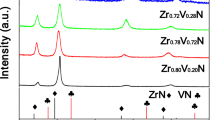
Figure 2a shows the XRD patterns of VN thin films deposited at different N2 flow rates of 5, 10, 15, 20 and 25 sccm. At 5sccm, a mixture of fcc-VN and hcp-V2N phases was obtained. The VN peaks at 36.95° and 42.08° correspond to (111) and (200) planes, respectively (JCPDS file 073–0528).The other diffraction peaks of V2N (111) and (200) at 39.91° and 41.07°, respectively (JCPDS file 032–1413), were also detected. With an increasing nitrogen flow to 10 sccm, in addition to V2N peaks, was noticed the fcc-VN phase at 43.48° showing a (200) predominate plane with other peaks at 37.48°, 62.72° and 74.95° corresponding to (111), (220) and (311) planes, respectively. At the same time, hcp-V2N phase disappeared which is in an agreement with XPS observations. The changes in the intensity were qualitatively estimated in terms of texture coefficients (TC) calculated from the XRD peaks by using Eq. (1). Figure 2b shows the (111) (200) and (220) texture coefficients of VN thin films as a function of N2 flow rate. At 5 sccm of N2, the texture coefficient of (111) orientation was 0.43 which corresponds to a random orientation of fcc-VN indicated in the JCPDS data file. With an increase of N2 flow rate up to 10 sccm, VN film with (200) dominate orientation exhibited a higher texture coefficient in comparison to the (111) orientation. Further rising in N2 flow to 15 and 20 sccm led to VN films with lower (200) texture coefficient and higher (111) orientation texture coefficient, respectively. The significant increase in the (111) intensity as compared to (200) plane intensity can be attributed to the competition between the surface energy and the strain energy with decreasing film thickness. A high intensity of (200) plane is observed with TC of 0.99 and a ratio of 78% and its 2θ value is well correlated to the (JCPDS file 073–0528) for the VN thin films. The high intensity of (200) plane of VN thin films is due to the low lattice strain (ɛ = -0.00197) [28]. The XRD spectra of VN films produced under further increase in N2 flow rate (15 and 20 sccm) reveal higher peak intensity of (111) plane with a maximum value of TC (2.2) and ratio of 78% and lower peak intensity of (200) plane (TC = 1.4, ratio of 25%) with a significant shift of 2θ angles was observed of the (220) plane (TC = 1.1), which despaired at higher nitrogen flow rate (Fig. 2b). According to Eq. (2), we know that the (111) and (002) planes were the preferential crystallite orientation for the thin VN films. The TC (h k l) represents the texture of a particular plane, whose deviation from unity implies the preferred growth [28]. The highest TC was in the (111) plane and a maximum ratio of 75% for thin VN film at 20 sccm [21]. The highest TC in the (111) plane is the result of an increasing lattice strain by the insertion of more nitrogen into the vanadium [28]. Huang et al. reported the same tendency of texture transition between (200) and (111) of VN films with increasing nitrogen partial pressure, which is predicted by the competitive growth theory [20].
As listed in Table 1, the lattice strain in the VN thin films, deposited under different N2 flow rates, is in the range from −0.00442 to 0.00052. This has a significant effect on the increase in the (111) plane intensity due to the grown stress, which is associated with the film thickness. The plane intensity evolution may also be related to the stoichiometry and chemical composition of the VN film. For the under-stoichiometric VN thin film (N/V < 1), there are fewer V–N bonds as compared to V-V bonds and hence (200) orientation. In the case of stoichiometric composition of VN thin film (N/V ≈ 1), the number of V–N bonds increased favoring the (111) plane [35].
As shown in Table 1, the increasing of lattice parameter of VN thin film from 0.41221 to 0.41416 nm with increasing N2 flow rate from 5 to 25 sccm can be clearly noticed. The average lattice parameter of the stoichiometric VN thin film (deposited at 20 sccm of N2) was equal to 0.41396 nm, which is in a good agreement with the theoretical value (0.41391 nm) indicated in the JCPDS file 073–0528. Thus, the results reveal an increase in lattice parameter of fcc-VN phase, which is in an agreement with the literature [36, 37]. According to our previous work [21], the rising of lattice parameter may be attributed to the increase of N/V ratio in VN thin films.
Surface topography and film morphology
The surface and cross-sectional morphology of VN thin films deposited at 10, 15 and 20 sccm of N2 flow rates are shown in Fig. 3. As the flow rate is increased from 5 to 10 sccm (Fig. 3a, b), the film surface morphology shows a faceted and a relatively rough surface (Ra = 25–37 nm). The cross-sectional image shows a columnar structure (with a film thickness of 1240 nm) as shown in Fig. 3a, b. Figure 3c shows that increasing nitrogen flow rate to 15 sccm results in are duction in the grain size separated by voids. Reduction in both crystallite size and surface roughness is attributed to the dense structure and the smooth surface features. At 20 sccm of N2, a completely different evolution was found where a columnar structure consisted of non-separable grains with apparently globular-like features (Fig. 3d). Furthermore, the structure was denser with a smoother surface (Ra = 6 nm) as a result of the fcc-VN phase stability (N/V≈1). According to Zhou et al. [38], nitrogen plays an important role in the film smoothening due to the presence of more nuclei. By increasing nitrogen flow rate, the thickness of the VN thin film decreased with no significant difference in the film growth morphology. This is due to the increasing density of gas molecules with increasing N2 flow rate that leads to more collisions and consequently a reduction in the film thickness [39].
Furthermore, the sputtering rate of nitrides is lower than that of metals and the metal nitride by the poisoning of the target at high nitrogen flow, which further decreases the sputtering yield of the targets [38]. The contamination of the vanadium target by nitrogen can also be another reason for the reduction in the film thickness [20, 38]. At higher nitrogen flow rate, the surface roughness slightly increased with an increase in grain size and a decrease in the film thickness (Fig. 3e).
Mechanical properties
Residual stress measurements
Figure 4 shows the evolution of residual stress in the films deposited at different N2 flow rates. In all VN thin films, a compressive stress state was observed. By increasing the N2 flow rate from 5 to 15 sccm, the residual stress increased about three times from -0.54 to-1.7 GPa. This can be related to the high density of the grain boundaries and defects as well as the coexistence of VN and V2N phases as confirmed by XRD and XPS analyses [40, 41]. From Fig. 4, we can see that, a further increase in the N2 flow rate to 20 sccm did not lead to any changes in the stress state, which is related to the progressive appearance of more stable VN phase [42]. The VN film deposited at 25 sccm of N2 presented lower stress where the VN phase was dominant. This is due to the reduction in the amount of interstitial defects by the incorporation of more nitrogen atoms in the vanadium lattice as well as the decrease of the crystalline size [41].
Hardness and elastic modulus
The hardness (H) and Young’s modulus (E) of the VN thin films are presented in Fig. 5a. Depending on the N2 flow rate (the nitrogen content in the films), the hardness values are in the range of 11–26.2 GPa. Figure 5c shows clearly the relationship between the film hardness and residual stress.
It can be noticed that increasing the N2 flow rate led to an increase the hardness of VN thin films. Its maximum value of 26.2 GPa was obtained for the film deposited at 20 sccm of N2. It has been observed earlier by Caicedo et al. [17], that the lowest hardness of 11 GPa was obtained for VN films deposited without bias voltage presenting a N/V ratio equal to 1. With further increase in bias voltage, the N/V ratio increased up to 1.14 and the hardness was enhanced to reach a maximum value of 20 GPa. It is evident that at 25 sccm of N2, when the N/V ratio was 1.17, the film hardness slightly decreased to 25 GPa, which is lower than the value (29 GPa) obtained in our previous study [21]. According to Korneev et al. [43], an increase in film hardness is attributed to the solid solution strengthening by the formation of stoichiometric structure, high compressive stress and lattice distortions related to structural defects. Besides, the reduction of hardness may be due to the formation of vanadium oxide phase [21].
Influence of porosity on the measured Young’s modulus of the VN coatings was observed. Results suggest that the Young’s modulus of VN thin films is strongly dependent on the amount of porosity and type of microstructure. The Young’s modulus of the dense film at higher nitrogen flow rate is more than that of the film with a columnar structure. The decrease in Young’s modulus can be explained by the fact that the columnar structures become more porous, with feather-like structures and an increased number of intra-columnar pores in the columns, which is consistent with several works in the literature of the bulk materials [44, 45]. Moreover, the hardness of the VN films a dense structure is more than that of the film with columnar structures in the surface regions of the coatings. This distinct difference in behavior of the hardness and Young’s modulus with film density in the VN thin films shows that the columnar structure produces anisotropic mechanical properties that are very different to those of their dense compact.
According to the literature, (H/E) and (H3/E2) are very important parameters [21, 47]. The H/E ratio can be used as an indicator for the elastic behavior of the surface in contact with external forces and the plasticity index (H3/E2) is reported to be a close measure of toughness, which allows the determination of elastic and plastic deformation resistance and rank the coatings accordingly [45]. The values of (H/E) and (H3/E2) calculated from hardness and Young modulus are shown in Fig. 5b. By increasing the N2 flow rate, the highest values of H/E (0.124) and H3/E2 (0.474 GPa) ratios were obtained in the film sputtered at 20 sccm (Fig. 5b), which indicate a greater toughness and resistance to abrasion.
The increase in the elasto-plastic index with higher hardness can be explained by the high dense structure and the high (111) textured plane due to the incorporation of nitrogen in the vanadium lattice and the formation of the VN phase, which is denser in comparison to the V2N [21, 46]. Moreover, the best mechanical is due to the high content of V–N binging energy and solid solution hardening from the nitrogen atoms in the VN films as compared to the pure vanadium, which is consistent with several works in the literature [46, 47].
Figure 5d shows the variation of hardness of the VN thin films as a function of the crystallite size of VN phase. This figure reveals an inverse relationship between the crystallite size and hardness, suggesting that a higher crystallite size corresponds to lower hardness. Hence, it can be inferred that increasing the nitrogen content helps in the grain refining of the VN film by the formation of more interfaces which block the dislocations movement, and thus strengthening the films, as described by Hall–Petch relationship [41, 49]. It has been shown by Huang et al. [20] that at N/V ratio = 1, the crystallite size was about 15 nm and the texture coefficients were 0.35 (111) and 0.65 (200) corresponding to 30 GPa super hard VN film. In the present study, the minimum crystallite size of 25 nm was obtained in film containing 49.5 at.% of nitrogen (N/V ratio close to 1), deposited at 20 sccm of N2, with a maximum average hardness of 26.2 GPa.
The Young's modulus of VN thin films was found to be between 164 and 226 GPa. This is considerably lower than the value (370 GPa) obtained by Huang et al. for VN thin film deposited by an unbalanced magnetron sputtering [20]. This significant difference may be attributed to the influence of high compressive stress, grain refinement and lower surface roughness, playing an important role in the nanoindentation measurements [2, 21]. The low Young's modulus values can also be explained by the increase of the fcc-VN phase fraction and the high residual porosity in the film because of the columnar structure [50]. According to Leyland et al. [30], the combination of high hardness and low Young's modulus enhances the film adhesion to substrate and provides better wear resistance.
Friction behavior and wear resistance
Figure 6a illustrates the friction coefficients and wear rates for all VN thin films deposited on XC100 steel substrates tested against 100Cr6 balls at room temperature under dry conditions. In the initial stage of sliding, a low value of friction coefficient (CoF) is observed, which then rises rapidly to a constant level after approximately 25 m of sliding distance, representing a transition pattern of friction for rough surfaces. According to our previous study [21], the low CoF value in the initial stages of the sliding test is due to the high surface roughness resulting in a small contact area between the film surface and the ball.
The average friction coefficient of the VN thin film deposited at 5 sccm of N2 was 0.61. This high value can be related to the hcp-V2N phase [21]. The VN film containing 49.5 at.% of nitrogen, deposited at 20 sccm of N2, presented the lowest friction coefficient of 0.37. Zhang et al. [50] reported a similar mechanism obtained during wear testing of sputtered TiN coatings used for synchronizer ring. Change in friction coefficient might be related to the formation of oxide phase (V2O5) on the surface of VN coating deposited at 20 sccm. Presence of this phase on the surface was confirmed by XPS analysis. V2O5 phase along with other V–O phases (VnO2n-1 and VnO3n-1) exhibit remarkable properties including oxidation stability, low adhesion as well as self-lubrication due to availability of easy sharable crystallographic defect planes [32].
The calculated wear rates of the VN thin films are illustrated in Fig. 6b. The wear rates of all VN thin films are lower than those of the VN films deposited by cathodic arc ion plating method due to the lubrication effect of vanadium oxide formed in VN films [51]. The wear rate of the VN films decreased with increasing nitrogen flow rate, signifying an enhancement in their wear resistance. The lowest wear rate of 2.72 × 10–5 mm3/N∙m was obtained for the VN film deposited at 20 sccm of N2. In order to describe the wear characteristics more clearly, SEM and two-dimensional profilometer images of wear tracks were acquired (Figs. 7 and 8). For the VN film deposited at 10 sccm of N2, the wear track was seriously worn and a delamination fracture of the VN thin film from the XC100 substrate was observed with a wear track depth of about 0.80 µm, indicating that the film has undergone severe abrasive wear. The wear debris produced during this abrasive wear could have further caused severe three-body wear at the interface resulting in increased wear (Fig. 7b). Between 5 and 10 sccm of N2, the SEM images of the wear tracks show the presence of significant debris around the edges with a pronounced wear track depth (Figs. 7a, b, 8a, b). Thus, this may have significantly contributed to a noticeable increase in the friction coefficient value between the surfaces of the VN film and the 100Cr6 ball [52].
For the films deposited at 15 and 20 sccm of N2, the wear track depths were about 0.75 and 0.70 µm, respectively, signifying the fact that increasing nitrogen content in the films enhances the film wear resistance [1]. Moreover, the wear tracks revealed a small amount of debris that indicating an improved tribological performance of the films with increasing nitrogen content in the VN thin films (Fig. 7c, d). The V–N coatings deposited at 15 and 20 sccm of N2 confirm increased wear resistance with lower wear rates (Fig. 8b, c), which is attributed to their dense structure, low surface roughness and high hardness value [21, 52].
EDS analysis was conducted to evaluate the elemental compositions on the wear track after the tests as shown in Fig. 9. The wear tracks on the VN thin films deposited at 5, 10 and 25 sccm flow rates of N2 showed the presence of iron peak, corresponding to the XC100 steel substrate with a small amount V and N. The high iron concentrations in worm track of the VN thin films indicate low films/substrate adhesion due to the low film thicknesses which affect negatively on the wear rate and friction coefficient values. However, the high V and N were noticed in case of the VN films deposited at 20 sccm of N2 flow rate, suggesting a stronger adhesion between the film and the substrate due to the dense structure and good mechanical properties of the film.
Improving the mechanical properties in terms of enhanced hardness, Young’s modulus and H/E and H3/E2 ratios of the VN films, results in increased resistance to brittle cracking, which is directly related to the tribological performance of the films. Hence, the worn surface of the films deposited at 5 sccm of N2, with lower H/E and H3/E2 values, shows poor tribological performance with brittle features (Fig. 8c. The worn surface of VN thin film at 20 sccm of N2 with higher H/E and H3/E2 shows the lowest wear rate (Fig. 6c. Moreover, it is also to be noted that many other variables may influence wear behavior such as crystal structure, grain refinement and reactivity between the coating and sliding material. The Hall–Petch strengthening with decrease in grain size leads to enhanced yield strength of the material and increases the hardness. It also blocks the propagation of cracks and dislocations and avoids the continuity of pores or pinholes in the deposited films [21]. Few studies have recently reported on the beneficial impact of nitrogen flow rate on crack deflections in TiN coatings [46].
Conclusions
In the present work, VN thin films were deposited on XC100 steel substrates and Si wafers by R.F. magnetron sputtering technique. The relationship between residual stress, lattice, grain size, phases and tribo-mechanical properties was illustrated. With increasing nitrogen content, coating composition changed from a mixture of V2N and VN phases to pure VN. The high intensity of (200) plane was obtained in films containing low nitrogen concentration, while the high intensity of (111) plane was remarkable in VN films deposited at higher nitrogen flow rate, which attributed to increase in the residual stress, and the film stoichiometry.
All films showed columnar structure. The addition of nitrogen clearly resulted in the formation of smooth films with fine grains. The average roughness of VN film was reduced from 25 to 6 nm with increasing N2 flow rate. Mechanical and tribological properties of VN thin films were considerably improved, and VN film deposited at 20 sccm of N2 had optimized properties. This film presented the highest hardness, lowest wear rate and a good friction coefficient of 0.37.
References
Ermolenko M-V, Zavadski S-M, Golosov D-A, Melnikov S-N, Zamburg E-G (2016) Tribologicalbehavior of TiN films deposited by reactive magnetron sputtering under low pressure. J Friction Wear 37(3):289–292
Aissani L, Nouveau C, Walock MJ, Djebaili H, Djelloul A (2015) Influence of vanadium on structure, mechanical and tribological properties of CrN coatings. Surf Eng 31(10):779–788
Krishna M-G, Bhattacharya AK (1999) Optical and electrical properties of vanadium nitride thin films. Int J Mod Phys B 13(7):833–839
C.-Y. Pu, D.-W. Zhou, D.-X. Bao, C. Lu, X.-L. Jin, T.-C. Su, F.-W. Zhang, Elastic and thermodynamic properties of vanadium nitride under pressure and the effect of metallic bonding on its hardness, Chinese Physics B 23(2) (2014) (026201) 1–6
Huber Š, Jankovský O, Sedmidubský D, Luxa J, Klímová K, Hejtmánek J, Sofer Z (2016) Synthesis, structure, thermal, transport and magnetic properties of VN ceramics. Ceram Int 42(16):18779–18784
Kwon H, Choi S, Thompson L-T (1999) Vanadium nitride catalysts: synthesis and evaluation forn-butane dehydrogenation. J Catal 184(1):236–246
Wiklund U, Casas B, Stavlid N (2006) Evaporated vanadium nitride as a friction material in dry sliding against stainless steel. Wear 261(1):2–8
Chun S-Y (2017) Properties of VN coatings deposited by ICP assisted sputtering: effect of ICP power. J Korean Ceram Soc 54(1):38–42
Ouldhamadouche N, Achour A, Lucio-Porto R, Islam M, Solaymani S, Arman A, Ahmadpourian A, Achour H, Brizoual L, Djouadi MA, Brousse T (2018) Electrodes based on nano-tree-like vanadium nitride and carbon nanotubes for micro-supercapacitors. J Mater Sci Technol 34:976–982
Zhang J, Li X, Dong X, Dong H, Oganov A-R (2020) Theoretical study of the pressure-induced structure, phase transition, mechanical and electronic properties in the V-N system. cond-mat.mtrl-sci., preprint, arXiv:2003.00036
Hofer E, Warbichler P, Scott A, Brydson R, Galesic I, Kolbesen B, Microsc J (2001) Electron energy loss near edge structure on the nitrogen K-edge in vanadium nitrides. J Microscopy 204:166–171
Wiemer C, SanjineÂs R, LeÂvy F (1996) Deposition and characterization of refractory ternary phases: the transition metal nitride Ti1−xMoxNy. Surf Coat Technol 86:372–376
Aperador W, Duque J, Delgado E (2016) Mechanical, Microstructural and tribo-electrochemistry characterization in aqueous media of coatings vanadium/vanadium nitride used as functional coating for implants. Int J Electrochem Sci 11:4688–4700
Navarro-Devia J-H, Amaya C, Caicedo J-C, Aperador W (2017) Performance evaluation of HSS cutting tool coated with hafnium and vanadium nitride multilayers, by temperature measurement and surface inspection, on machining AISI 1020 steel. Surf Coat Technol 332:484–493
Chu X, Barnett S-A, Wong M-S, Sproul W-D (1996) Reactive magnetron sputter deposition of polycrystalline vanadium nitride films. J Vac Sci Technol, A 14(6):3124–3129
Suszko T, Gulbiński W, Urbanowicz A (2011) Preferentially oriented vanadium nitride films deposited by magnetron sputtering. Mater Lett 65(14):2146–2148
Caicedo J-C, Zambrano G, Aperador W, Alarcon L-E, Camps E (2011) Mechanical and electrochemical characterization of vanadium nitride (VN) thin films. Appl Surf Sci 258(1):312–320
Qiu Y, Zhang S, Li B, Lee J-W, Zhao D (2012) Influence of nitrogen partial pressure and substrate bias on the mechanical properties of VN coatings. Procedia Engineering 36:217–225
Toth H-L (2014) Transition metal carbides and nitrides. Elsevier Science
Huang J-H, Lin C-H, Yu G-P (2019) Texture evolution of vanadium nitride thin films. Thin Solid Films 688(137415):1–10
Aissani L, Alhussein A, Nouveau C, Ghelani L, Zaabat M (2019) Influence of film thickness and Ar-N2 plasma gas on the structure and performance of sputtered vanadium nitride coatings. Surf Coat Technol 378(25):124948
Huang J-H, Lau K-W, Yu G-P (2005) Effect of nitrogen flow rate on structure and properties of nanocrystallineTiN thin films produced by unbalanced magnetron sputtering. Surf Coat Technol 191:17–24
Warren B-E (1969) X-ray Diffraction, Addison Wesley Publishing Co., London p. 381
Greczynski G, Hultman L (2020) X-ray photoelectron spectroscopy: towards reliable binding energy referencing. Progress Mater Sci 107:100591
Zheng X-H, Walmsley D-G (2015) Discrepancy between theory and measurement of superconducting vanadium. Physica C 515:41–48
Lin Y-W, Lu C-W, Yu G-P, Huang J-H (2016) Structure and properties of nanocrystalline (TiZr)xN1−x thin films deposited by DC unbalanced magnetron sputtering. J Nanomater 2016:2982184
Aissani L, Fellah M, Radjehi L, Nouveau C, Montagne A, Alhussein A (2019) Effect of annealing treatment on the microstructure, mechanical and tribological properties of chromium carbonitride coatings. Surf Coat Technol 359:403–413
Pelleg J, Zevin L-Z, Lungo S, Croitoru N (1991) Reactive-sputter-deposited TiN films on glass substrates. Thin Solid Films 197:117–128
Liao M-J, Gotoh Y, Tsuji H, Ishikawa J (2004) Crystallographic structure and composition of vanadium nitride films deposited by direct sputtering of a compound target. J Vac Sci Technol A 22:146–150
Sanjinés R, Hones P, LeÂvy F (1998) Hexagonal nitride coatings: electronic and mechanical properties of V2N, Cr2N and d-MoN. Thin Solid Films 332:225–229
SanjineÂs R, Hones P, LeÂvy F (1998) Hexagonal nitride coatings: electronic and mechanical properties of V2N, Cr2N and d-MoN. Thin Solid Films 332:225–229
Glaser A, Surnev S, Netzer FP, Fateh N, Fontalvo GA, Mitterer C (2007) Oxidation of vanadium nitride and titanium nitride coatings. Surf Sci 601:1153–1159
Osonkie V, Chukwunenye PL, Cundari T, Kelbera J (2020) Plasma modification of vanadium oxynitride surfaces: characterization by in situ XPS experiments and DFT calculations. J Chem Phys 153(144709):1–8
Liu H-H, Zhang H-L, Xu H-B, Lou T-P, Sui Z-T, Zhang Y (2018) Hierarchically nanostructured vanadium nitride microspheres assembled with porous nanosheets fabricated by a template-free route. Ceram Int 44(2):1583–1588
A. Nelkel, The Physics and Chemistry of Carbides; Nitrides and Borides, Kluwer Academic Publishers, Netherlands in: R. Freer (Ed.) (1990) 279–296
Mei A-B, Hellman O, Wireklint N, Schleputz CM, Sangiovanni DG, Alling B, Rockett A, Hultman L, Petrov I, Greene JE (2015) Dynamic and structural stability of cubic vanadium nitride. Phys Rev B 91(054101):1–11
Isaev EI, Simak S-I, Abrikosov I-A, Ahuja R, Vekilov Y-K, Katsnelson MI, Lichtenstein A-I, Johansson B (2007) Phonon related properties of transition metals, their carbides, and nitrides: a first-principles study. J Appl Phys 101:123519
Forniés E, Galindo R-E, Sánchez O, Albella J (2006) Growth of CrNx films by DC reactive magnetron sputtering at constant N2/Ar gas flow. Surf Coat Technol 200:6047–6053
Li D, Chen J, Zou C, Ma J, Li P, Li Y (2014) Effects of Al concentrations on the microstructure and mechanical properties of Ti–Al–N films deposited by RF-ICPIS enhanced magnetron sputtering. J Alloy Compd 609:239–243
Aissani L, Alhussein A, Nouveau C, Radjehi L, Lakdhar I, Zgheib E (2019) Evolution of microstructure, mechanical and tribological properties of vanadium carbonitride coatings sputtered at different nitrogen partial pressures. Surf Coat Technol 374:531–540
Bouaouina B, Besnard A, Abaidia S-E, Airoudj A, Bensouici F (2018) Correlation between mechanical and microstructural properties of molybdenum nitride thin films deposited on silicon by reactive R.F. magnetron discharge. Surf Coat Technol 333:32–38
Detor A-J, Hodge A-M, Chason E, Wang Y, Xu H, Conyers M, Nikroo A, Hamza A (2009) Stress and microstructure evolution in thick sputtered films. Acta Mater 57:2055–2065
Schulz U, Terry SG, Levi G (2003) Microstructure and texture of EB-PVD TBCs grown under different rotation modes. Mater Sci Eng A 360:319–329
Singh J, Wolfe DE, Singh J (2002) Architecture of thermal barrier coatings produced by electron beam-physical vapor deposition (EB-PVD). J Mater Sci 37:3261–3267. https://doi.org/10.1023/A:1016187101616
Korneev P-V-K, Pierson J-F, Petrzhik M-I, Alnot M, Levashov E-A, Shtansky D-V (2009) Effect of nitrogen partial pressure on the structure, physical and mechanical properties of CrB2 and Cr–B–N films. Thin Solid Films 517:2675–2680
Lv Y, Ji L, Liu X, Li H, Zhou H (2012) Influence of substrate bias voltage on structure and properties of the CrAlN films deposited by unbalanced magnetron sputtering. Appl Phys Lett 258:3864–3870
Puneet C, Valleti K, Gopal AV, Joshi SV (2018) CrAlSiN nanocomposite thin films for high-speed machining applications. Mater Manuf Process 33(4):371–377
Aissani L, Alhussein A, Ayad A, Nouveau C, Zgheib E, Belgroune A, Zaabat M, Barille R (2021) Relationship between structure, surface topography and tribo-mechanical behavior of Ti-N thin films elaborated at different N2 flow rates. Thin Solid Films 724:138598
Aissani L, Alhussein A, Belgroune A, Nouveau C, Zgheib E, Barille R, Montagne A (2021) Effect of carbon content on structural, mechanical and tribological properties of Cr-V-C-N coatings. Thin Solid Films 732:138782
Zhang S, Yan F, Yang Y, Yan M, Zhang Y, Guo J, Li H (2019) Effects of sputtering gas on microstructure and tribological properties of titanium nitride films. Appl Surf Sci 488:61–69
Mu Y, Liu M, Zhao Y (2016) Carbon doping to improve the high temperature tribological properties of VN coating. Tribol Int 97:327–336
M.-H. Staia, A. Trocelis, A. Zairi, M. Suarez, E. S. Puchi-Cabrera, A. Iost, A. Montagne, Assessment of the mechanical and tribological performance of a ZrN PVD coating, Surf. Eng.(2017) 491–500
Acknowledgements
The authors gratefully acknowledge the LaBoMaP of Arts et Metiers Institute of Technology in Cluny for their help in the deposition and characterization of coatings.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Catalin Croitoru.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aissani, L., Fellah, M., Chadli, A.H. et al. Investigating the effect of nitrogen on the structural and tribo-mechanical behavior of vanadium nitride thin films deposited using R.F. magnetron sputtering. J Mater Sci 56, 17319–17336 (2021). https://doi.org/10.1007/s10853-021-06393-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06393-0