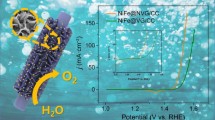
Abstract
Overall urea electrolysis has favorable thermodynamic properties and can complement traditional water splitting by simultaneously purifying urea-rich waste streams. However, the kinetics of the reaction is slow and more efficient catalysts are required. In this work, we describe a facile way to fabricate amorphous nickel phosphides on graphene nanosheets (a-Ni2P/G), which shows high efficient performance for overall urea electrolysis. The a-Ni2P/G catalyst has abundant surface area for abundant catalytic active sites and high proportion of Ni3+ active sites, realizing a superior intrinsic activity for both hydrogen evolution reaction (HER) and urea oxidation reaction (UOR). Impressively, the a-Ni2P/G catalyst requires only much lower potentials of 1.28 V and − 0.1 V for UOR and HER at 10 mA cm−2. The assembled a-Ni2P/G║a-Ni2P/G electrolyzer needs a small cell voltage of 1.39 V to deliver the current density of 10 mA cm−2. This work highlights a promising pathway to develop a cost-efficient catalyst for energy-saving H2 generation combined with waste water purification.
Graphical abstract
A highly efficient amorphous catalyst of a-Ni2P/G with high surface area and abundant exposed catalytic sites is reported. The assembled a-Ni2P/G║a-Ni2P/G system shows a quite small cell voltage of 1.39 V to deliver 10 mA cm−2, which is better than most of bifunctional catalysts.







Similar content being viewed by others
References
Ormerod RM (2003) Solid oxide fuel cells. Chem Soc Rev 32:17–28
Mallouk TE (2013) Divide and conquer. Nat Chem 5:362–363
Senthilkumar N, Gnana Kumar G, Manthiram A (2018) 3D hierarchical core–shell nanostructured arrays on carbon fibers as catalysts for direct urea fuel cells. Adv Energy Mater 8:1702207
Boggs BK, King RL, Botte GG (2009) Urea electrolysis: direct hydrogen production from urine. Chem Commun 32:4859–4861
Lan R, Tao S, Irvine JTS (2010) A direct urea fuel cell—power from fertiliser and waste. Energy Environ Sci 3:438–441
Tong Y, Mao H, Xu Y, Liu J (2019) Oxygen vacancies confined in Co3O4 quantum dots for promoting oxygen evolution electrocatalysis. Inorg Chem Front 6:2055–2060
Suen N-T, Hung S-F, Quan Q, Zhang N, Xu Y-J, Chen HM (2017) Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem Soc Rev 46:337–365
Tong Y, Mao H, Chen P, Sun Q, Yan F, Xi F (2020) Confinement of fluorine anions in nickel-based catalysts for greatly enhancing oxygen evolution activity. Chem Commun 56:4196–4199
Wang C, Lu H, Mao Z, Yan C, Shen G, Wang X (2020) Bimetal Schottky heterojunction boosting energy-saving hydrogen production from alkaline water via urea electrocatalysis. Adv Funct Mater 30:2000556
Zhu B, Liang Z, Zou R (2020) Designing advanced catalysts for energy conversion based on urea oxidation reaction. Small 16:1906133
Tong Y, Chen P, Zhang M, Zhou T, Zhang L, Chu W, Wu C, Xie Y (2018) Oxygen vacancies confined in nickel molybdenum oxide porous nanosheets for promoted electrocatalytic urea oxidation. ACS Catal 8:1–7
Yu Z-Y, Lang C-C, Gao M-R, Chen Y, Fu Q-Q, Duan Y, Yu S-H (2018) Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Energy Environ Sci 11:1890–1897
Chen S, Duan J, Vasileff A, Qiao SZ (2016) Size fractionation of two-dimensional sub-nanometer thin manganese dioxide crystals towards superior urea electrocatalytic conversion. Angew Chemie Int Ed 55:3804–3808
Li F, Chen J, Zhang D, Fu W-F, Chen Y, Wen Z, Lv X-J (2018) Heteroporous MoS2/Ni3S2 towards superior electrocatalytic overall urea splitting. Chem Commun 54:5181–5184
Zhu W, Ren M, Hu N, Zhang W, Luo Z, Wang R, Wang J, Huang L, Suo Y, Wang J (2018) Traditional NiCo2S4 phase with porous nanosheets array topology on carbon cloth: a flexible, versatile and fabulous electrocatalyst for overall water and urea electrolysis. ACS Sustain Chem Eng 6:5011–5020
Wang L, Ren L, Wang X, Feng X, Zhou J, Wang B (2018) Multivariate MOF-templated pomegranate-like Ni/C as efficient bifunctional electrocatalyst for hydrogen evolution and urea oxidation. ACS Appl Mater Interfaces 10:4750–4756
Li Y, Tan X, Chen S, Bo X, Ren H, Smith SC, Zhao C (2019) Processable surface modification of nickel-heteroatom (N, S) bridge sites for promoted alkaline hydrogen evolution. Angew Chemie Int Ed 58:461–466
Wang T, Wang M, Yang H, Xu M, Zuo C, Feng K, Xie M, Deng J, Zhong J, Zhou W et al (2019) Weakening hydrogen adsorption on nickel via interstitial nitrogen doping promotes bifunctional hydrogen electrocatalysis in alkaline solution. Energy Environ Sci 12:3522–3529
Tong Y, Wu J, Chen P, Liu H, Chu W, Wu C, Xie Y (2018) Vibronic superexchange in double perovskite electrocatalyst for efficient electrocatalytic oxygen evolution. J Am Chem Soc 140:11165–11169
Daramola DA, Singh D, Botte GG (2010) Dissociation rates of urea in the presence of NiOOH catalyst: a DFT analysis. J Phys Chem A 114:11513–11521
Shen F, Jiang W, Qian G, Chen W, Zhang H, Luo L, Yin S (2020) Strongly coupled carbon encapsulated Ni-WO2 hybrids as efficient catalysts for water-to-hydrogen conversion via urea electro-oxidation. J Power Sources 458:228014
Xu Q, Qian G, Yin S, Yu C, Chen W, Yu T, Luo L, Xia Y, Tsiakaras P (2020) Design and synthesis of highly performing bifunctional Ni-NiO-MoNi hybrid catalysts for enhanced urea oxidation and hydrogen evolution reactions. ACS Sustain Chem Eng 8:7174–7181
Wang D, Lu J, Luo L, Jing S, Abbo HS, Titinchi SJJ, Chen Z, Tsiakaras P, Yin S (2019) Enhanced hydrogen evolution activity over microwave-assisted functionalized 3D structured graphene anchoring FeP nanoparticles. Electrochim Acta 317:242–249
Chen W, Qian G, Xu Q, Pan M, Luo L, Yin S (2021) Nitrogen-doped-carbon coated FeCo modified CoFe2O4 nanoflowers heterostructure with robust stability for oxygen evolution and urea oxidation. Electrochim Acta 371:137817
Qian G, Chen J, Luo L, Zhang H, Chen W, Gao Z, Yin S, Tsiakaras P (2020) Novel bifunctional V2O3 nanosheets coupled with N-doped-carbon encapsulated Ni heterostructure for enhanced electrocatalytic oxidation of urea-rich wastewater. ACS Appl Mater Interfaces 12:38061–38069
Wu F, Zhang Z, Zhang F, Duan D, Li Y, Wei G, Liu S, Yuan Q, Wang E, Hao X (2018) Exploring the role of cobalt in promoting the electroactivity of amorphous Ni-B nanoparticles toward methanol oxidation. Electrochim Acta 287:115–123
Xie J, Qu H, Lei F, Peng X, Liu W, Gao L, Hao P, Cui G, Tang B (2018) Partially amorphous nickel-iron layered double hydroxide nanosheet arrays for robust bifunctional electrocatalysis. J Mater Chem A 6:16121–16129
Zhang H, Chen B, Jiang H, Duan X, Zhu Y, Li C (2018) Boosting water oxidation electrocatalysts with surface engineered amorphous cobalt hydroxide nanoflakes. Nanoscale 10:12991–12996
Zhang LY, Ouyang Y, Wang S, Wu D, Jiang M, Wang F, Yuan W, Li CM (2019) Perforated Pd nanosheets with crystalline/amorphous heterostructures as a highly active robust catalyst toward formic acid oxidation. Small 15:1904245
Nakamura N, Terban MW, Billinge SJL, Reeja-Jayan B (2017) Unlocking the structure of mixed amorphous-crystalline ceramic oxide films synthesized under low temperature electromagnetic excitation. J Mater Chem A 5:18434–18441
Yu J, Pan S, Zhang Y, Liu Q, Li B (2019) Facile synthesis of monodispersed α-Ni(OH)2 microspheres assembled by ultrathin nanosheets and its performance for oxygen evolution reduction. Front Mater 6:124
Feng Y, Xu C, Hu E, Xia B, Ning J, Zheng C, Zhong Y, Zhang Z, Hu Y (2018) Construction of hierarchical FeP/Ni2P hollow nanospindles for efficient oxygen evolution. J Mater Chem A 6:14103–14111
Li Y, Zhang H, Jiang M, Zhang Q, He P, Sun X (2017) 3D Self-supported Fe-doped Ni2P nanosheet arrays as bifunctional catalysts for overall water splitting. Adv Funct Mater 27:1702513
Huang Z, Chen Z, Chen Z, Lv C, Meng H, Zhang C (2014) Ni12P5 nanoparticles as an efficient catalyst for hydrogen generation via electrolysis and photoelectrolysis. ACS Nano 8:8121–8129
Yao Q, Zhou X, Xiao S, Chen J, Abdelhafeez IA, Yu Z, Chu H, Zhang Y (2019) Amorphous nickel phosphide as a noble metal-free cathode for electrochemical dechlorination. Water Res 165:114930
Morales C, Urbanos FJ, del Campo A, Leinen D, Granados D, Rodríguez MA, Soriano L (2020) Electronic decoupling of graphene from copper induced by deposition of ZnO: A complex substrate/graphene/deposit/environment interaction. Adv Mater Interfaces 7:1902062
Moon J-S, Jang J-H, Kim E-G, Chung Y-H, Yoo SJ, Lee Y-K (2015) The nature of active sites of Ni2P electrocatalyst for hydrogen evolution reaction. J Catal 326:92–99
Chen N, Du Y-X, Zhang G, Lu W-T, Cao F-F (2021) Amorphous nickel sulfoselenide for efficient electrochemical urea-assisted hydrogen production in alkaline media. Nano Energy 81:105605
Liu Y-H, Hung C-H, Hsu C-L (2021) Electrochemical fabrication of carbon fiber-based nickel hydroxide/carbon nanotube composite electrodes for improved electro-oxidation of the urea present in alkaline solutions. Sep Purif Technol 258:118002
Guo F, Ye K, Du M, Huang X, Cheng K, Wang G, Cao D (2016) Electrochemical impedance analysis of urea electro-oxidation mechanism on nickel catalyst in alkaline medium. Electrochim Acta 210:474–482
Liu H, Zhu S, Cui Z, Li Z, Wu S, Liang Y (2021) Ni2P Nanoflakes for the high-performing urea oxidation reaction: linking active sites to a UOR mechanism. Nanoscale 13:1759–1769
Li Y, Jiang H, Cui Z, Zhu S, Li Z, Wu S, Ma L, Han X, Liang Y (2021) Spin state tuning of the octahedral sites in Ni–Co-based spinel toward highly efficient urea oxidation reaction. J Phys Chem C 125:9190–9199
Xia J, Dhaka K, Volokh M, Peng G, Wu Z, Fu Y, Caspary Toroker M, Wang X, Shalom M (2019) Nickel phosphide decorated with trace amount of platinum as an efficient electrocatalyst for the alkaline hydrogen evolution reaction. Sustain Energy Fuels 3:2006–2014
Chakrabarty S, Offen-Polak I, Burshtein TY, Farber EM, Kornblum L, Eisenberg D (2021) Urea oxidation electrocatalysis on nickel hydroxide: the role of disorder. J Solid State Electrochem 25:159–171
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21905251) and the Science Foundation of Zhejiang Sci-Tech University (ZSTU) under Grant No. 18062242-Y. Y. Tong is also partially supported by a fellowship from the China Scholarship Council (CSC) and the Swiss Government Excellence Scholarship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Handling Editor: Kyle Brinkman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tong, Y., Chen, L., Dyson, P.J. et al. Boosting hydrogen production via urea electrolysis on an amorphous nickel phosphide/graphene hybrid structure. J Mater Sci 56, 17709–17720 (2021). https://doi.org/10.1007/s10853-021-06391-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06391-2