Abstract
The present work shows that thermal expansion experiments can be used to measure the γʼ-solvus temperatures of four Ni-base single-crystal superalloys (SX), one with Re and three Re-free variants. In the case of CMSX-4, experimental results are in good agreement with numerical thermodynamic results obtained using ThermoCalc. For three experimental Re-free alloys, the experimental and calculated results are close. Transmission electron microscopy shows that the chemical compositions of the γ- and the γʼ-phases can be reasonably well predicted. We also use resonant ultrasound spectroscopy (RUS) to show how elastic coefficients depend on chemical composition and temperature. The results are discussed in the light of previous results reported in the literature. Areas in need of further work are highlighted.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ni-based single-crystal superalloys (SXs) are used to manufacture turbine blades, which operate at temperatures above 1000 °C. They have to withstand a load spectrum, which includes creep, thermal fatigue and hot corrosion. Creep strength, the resistance against a slow but continuous accumulation of strain, is of utmost importance. It is well known that SXs rely for their strength on a microstructure, which consists of sub-micrometer cuboidal γʼ-particles (crystal structure: ordered L12-phase; volume fraction: 70 vol.%) which are separated by thin γ-channels (crystal structure: fcc; volume fraction: close to 30 vol.%), e.g., [1,2,3,4]. The crystal structures of both phases are similar, thus that on cooling from high temperatures the ordered γʼ-particles can coherently precipitate in the γ-matrix. The lattice constants d of the two phases differ. In Ni-base single-crystal superalloys, one often finds: dγʼ < dγ. The associated lattice misfit results in an increase in elastic strain energy, e.g., [5, 6]. This misfit and some of its consequences, e.g., its effect on the shape of γʼ-particles, on Peach–Köhler forces acting on channel dislocations, on rafting and on the formation of interfacial dislocation networks, have been and are still being discussed in the literature, e.g., [7,8,9,10,11,12,13,14,15]. The ordered γʼ-particles represent obstacles to dislocation motion. They are not completely impenetrable, but it is more difficult for dislocations to move through the ordered γʼ-phase than through the fcc γ-channels. The art of SX design partly relies on making it as difficult as possible for dislocations to cut into the γʼ-phase to optimize creep strength. Making cutting difficult is associated with increasing antiphase boundary energies (APBs), e.g., [16,17,18,19,20], which result from one of the most prominent elementary mechanisms in crystal plasticity: the pairwise cutting of the γʼ-phase, e.g., [21,22,23]. While in the literature there are disagreements as far as specific aspects of this cutting process are concerned, SX researchers from academia and industry believe that it is desirable to design alloys with a high γʼ-volume fraction, a high γʼ-solvus temperature and a chemical composition, which gives rise to high planar fault energies which affect the physical nature of APBs and stacking faults. The γʼ-solvus temperature has been considered as especially important and has been highlighted as a reference temperature in many scientific and technological SX publications, e.g., [24,25,26,27,28,29,30,31].
The present work takes a closer look at the thermodynamic properties of four SXs, ERBO/1 (with Re, a CMSX-4 type alloy) and three ERBO/15 variants (Re-free, with higher levels of Mo, Ti and W). It uses resonant ultrasonic spectroscopy (RUS) and dilatometry to measure elastic stiffnesses and thermal expansion coefficients as a function of temperature. It has recently been shown how these four alloys differ in terms of creep properties [32, 33].
The present work investigates how much large (ERBO/1 vs. ERBO/15) and small (three ERBO/15 variants) variations in alloy composition affect their thermoelastic properties. First objective: The comparison of two different alloys (large variation in alloy compositions) helps in the overall effort to move toward a superalloy single-crystal technology, where expensive and strategic alloy elements like Re, which are known to provide a high creep strength, are replaced by other elements without jeopardizing mechanical strength. Elastic and creep properties are both important in this respect. It has been proposed that this can be achieved by increasing the levels of Mo, Ti and W [34]. Further, elastic coefficients are needed in high-temperature engineering to design components, which need to withstand thermal fatigue loading. Therefore, an effort is made in the present work to measure elastic coefficients. Second objective: A detailed understanding of the role of individual alloy elements can only be obtained, when the effect of one particular element is studied. The comparison of the three ERBO/15 variants helps in this respect. Third objective: In particular, the potential of high-resolution dilatometry as a method for determining high γʼ-solvus temperatures is explored. For this purpose, we compare experimental results for γʼ-solvus temperatures obtained by high-temperature dilatometry with theoretical ThermoCalc calculations [35]. The quality of the ThermoCalc predications is assessed by comparing its predictions for the chemical compositions of the γ- and γʼ-phases obtained using 3D atom probe tomography (3D-ATP) [36] and transmission electron microscopy (TEM) [32]. To establish high-resolution measurements of thermal expansion as a method for determining γʼ-solvus represents a significant progress in superalloy technology.
The results are discussed in light of previous work published in the literature. Areas, which require further research, are highlighted.
Materials, experiments and methods
Materials: In the present work, four materials are investigated. Their nominal chemical compositions are listed in Table 1. ERBO/1 is a CMSX-4 type of alloy, details on processing, multiple step heat treatment and microstructure have been published elsewhere [32, 33, 36, 37]. ERBO/15 is a low-density Re-free single-crystal Ni-base superalloy, which has been developed by Rettig et al. [34] using a numerical thermodynamic multi-criteria optimization method. In the present work, we compare ERBO/15 with two leaner ERBO/15 variants, which contain less W and less Mo (ERBO/15-W and ERBO/15-Mo).
The heat treatment details of the four investigated alloys are presented in Table 2. While ERBO/1 was heat-treated by Doncasters Precision Castings in Bochum, the heat treatments of the ERBO/15 variants were performed in a custom-built vacuum heat treatment furnace from Carbolite Gero of type LHTM 100–200/16 1G. Detailed information about the heat treatment procedure is documented in [32] and [36].
Electron probe microanalysis (EPMA) was performed using an electron probe microanalyzer SX 50 for ERBO/1 and a field emission electron microprobe of type SXFiveFE for ERBO/15 and its two derivates, both from Cameca. It is well known that during solidification, alloy elements of SXs can vary in their tendencies to partition to dendritic and interdendritic regions. Figure 1 presents the distributions of the elements Al, Ti, Mo and W in the microstructure of ERBO/15 in the as-cast condition (upper row, Fig. 1a–d) and after the homogenization heat treatment (lower row, Fig. 1e–h). The lower row of Fig. 1 shows that the large-scale chemical heterogeneity, associated with the partitioning tendencies of the alloy elements during solidification, can be lowered during the homogenization step (Table 2); however, it does not completely vanish as can be seen for W in Fig. 1h.
Scanning electron microscopy (SEM) investigations were performed using a Leo Gemini 1530 SEM from Carl Zeiss AG equipped with a field emission gun (FEG) operating at 12 kV and an inlens detector (working distance: 4.5mm, aperture: 30 mm). Figure 2 presents SEM micrographs of the eight material states, which were considered in the present work. The four as-cast materials states are presented in the upper row of Fig. 2. The fully heat-treated material states after the secondary aging treatment (Table 2) are shown in the lower row of Fig. 2.
Transmission electron microscopy (TEM) was used to analyze the local alloy chemistry of the two phases in the γ/γʼ-microstructure. TEM was performed using a JEOL JSM-6490 (for ERBO/1) and a Tecnai G2 F20 from FEI (for ERBO/15 and its derivates), both equipped with a FEG operating at 200 kV and an EDAX analysis system. The procedure, which was used to measure phase compositions in the three ERBO/15 variants, is illustrated in Fig. 3. The picture in the middle of Fig. 3 shows a STEM micrograph taken in a < 100 > direction (multiple beam contrast). EDX spectra were taken at five γ-channel positions (1–5, as indicated) and in the centers of the four adjacent γʼ-particles (positions 6–9). Each EDX spectrum was recorded with an electron beam diameter < 10 nm over a time period of 60 s. Compositions were calculated using the EDAX algorithm. The values for the two phases were obtained as the averages of the five channel measurements (analysis points 1 to 5) and the four particle measurements (analysis points 6 to 9). Examples of EDX spectra for the cross γ-channel (in orange, position 1) and for the upper right γʼ-particle (in green, position 7) are shown in the left and right diagrams as indicated.
Examples for TEM EDX spectra from γ-channels and γʼ-particles in ERBO/15. Center: STEM micrograph indicating nine locations where EDX measurements were taken. Left: EDX spectrum from γ-channel crossing (position 1 in central image). Right: EDX spectrum from γ’-phase (position 7 in central image). Elements partitioning to the γ-matrix and γʼ-cubes are highlighted in orange and green
For comparison, we use some of the local composition results reported by Parsa et al. [36], which were obtained by a local electrode atom probe (LEAP™ 3000X HR, Cameca Instruments) in voltage mode at a specimen base temperature of ∼65 K.
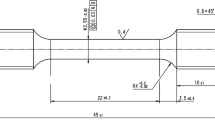
Resonant ultrasound spectroscopy (RUS): In the present work, elastic stiffness coefficients as a function of temperature were determined with the aid of resonant ultrasound spectroscopy (RUS), e.g. [38, 39]. Precisely < 100 > oriented cuboidal specimens of target dimensions of about 8 × 7 x 6 mm3 = 336 mm3 were obtained by combining Laue orientation with spark erosion machining as described in [40]. In the present work, RUS measurements were taken for ERBO/15 and its variants. For comparison, the data for ERBO/1 were taken from the work of Demtröder et al. [41]. All specimen dimensions are given in Table 3. For all specimens tested in the present work, there was good agreement between geometric densities \(\rho_{G}\) (calculated from sample weights and volumes) and the densities determined by the buoyancy method \(\rho_{B}\) (Table 3). Good surface qualities were established by grinding and diamond plate polishing.
In Fig. 4a, a typical RUS specimen is shown clamped in between two ceramic rods and the measurement thermocouple nearby. During the experiments, the thermocouple was not attached to the specimen but very close. Specimen and the relevant part of the thermocouple were in the temperature constant zone of the furnace.
For the experimental determination of resonance frequencies, freely vibrating samples are employed. The experimentally obtained resonance frequencies are related to the three independent elastic stiffness coefficients c11, c12 and c44 of the pseudo-cubic crystal [38, 39, 41]. For data collection, a RUS apparatus built in-house was used. The system consisted of a frequency response analyzer (type FRA5087 from NF Corporation) in combination with a high-speed amplifier (type BA4825 from NF Corporation) for signal generation and detection. The experiments were performed in the temperature range from 100 to 673 K using a Netzsch low-temperature furnace and a cascading temperature controller (type 2704 from Eurotherm). Resonance spectra of the samples were recorded in the range from 150 to 1000 kHz. In the case of ERBO/1, Demtröder et al. [41] studied the elastic properties in 50 K steps between room temperature and 1273 K. The ERBO/15 variants of the present work were investigated in 10 K steps between 100 and 673 K.
According to Demtröder et al. [41], the large-scale heterogeneities associated with the cast microstructure (dendrites and interdendritic regions) can influence the resonances with wave lengths of the order of the dendrite spacing. Following the procedure described by Demtröder et al. [41], elastic coefficients of all samples were refined on the basis of the 50 eigenmodes with the lowest frequencies (highlighted in gray in Fig. 5a–d) by a nonlinear least-squares fit procedure. Taking a closer look at the differences between the experimentally observed resonance frequencies fobs and those calculated from the refined sample parameters fcalc at room temperature yields deviations between 0.33 and 0.6 kHz on average, which documents the good quality of the refinements. In Fig. 5, these differences are plotted as a function of the eigenmodes from the lowest to the highest resonance frequency. Demtröder et al. [41] have shown that this difference increases with decreasing sample size. Most importantly, good results are obtained when the specimen dimensions exceed the mean spacing between dendrites by at least a factor of 10. The authors also concluded that average deviations of less than 2 kHz are acceptable. As can be seen in Fig. 5, the average scatter observed in the present work does not exceed this value.
Scatter of fcalc – fobs between calculated and observed resonance frequencies of the first 140 eigenmodes of freely vibrating samples: a ERBO/1. b ERBO/15. c ERBO/15-Mo. d ERBO/15-W. For details regarding sample parameters, see Table 3
Dilatometry (DIL): High-precision dilatometry was used to monitor the temperature dependence of the coefficient of thermal expansion αth. The thermally induced strain εth, i.e. the relative change of sample length ΔL / L0 (L0: sample length at 293 K) with temperature, was measured between 100 and 1573 K using an inductive gauge dilatometer of type DIL402c from Netzsch as described in [41]. As can be seen in Fig. 4b (specimen clamped in between two ceramic rods, thermocouple close but not yet attached), the specimens used for the measurement of thermal expansion had the same geometry and crystallographic orientation as those taken for the assessment of the elastic stiffnesses, see Table 3. The dilatometer was calibrated with standard samples of the same lengths made of corundum. All experiments were performed in He-atmosphere at heating rates of 2 K/min. The coefficients of linear thermal expansion \(\alpha_{th} = \partial {\kern 1pt} \varepsilon_{th} /\partial {\kern 1pt} T\) were determined as the first derivatives of the corresponding strain temperature curves. To this end, 40 (strain, temperature) data pairs within an interval of ± 1.5 K around each temperature Ti were approximated by a second-order polynomial from which \(\alpha_{th} (T_{i} )\) was calculated.
Thermodynamic calculations: In a multicomponent alloy, phase stabilities depend on alloy chemistry, temperature and pressure [43,44,45]. Today, the CALPHAD method (CALPHAD—short for: CALculation of PHAse Diagrams) originally developed by Kaufmann and Bernstein [45] can be used to calculate phase equilibria in multicomponent alloys [46, 47]. In the present work, ThermoCalc (a state-of-the-art CALPHAD implementation) in combination with database TCNi8, version 2019b [35]) was used to calculate thermodynamic equilibria with a focus on γʼ-solvus temperatures and the chemical compositions of the γ- and the γʼ- phases. Additionally, liquidus and solidus temperatures as well as the γʼ-volume fractions were calculated as a function of the temperature for all four alloys. These calculations are based on a homogeneous chemical distribution of alloy elements in our SX. In reality, there is a dendritic solidification with dendritic (D) and interdendritic (ID) regions containing different chemical compositions. However, as has been shown in [36], the differences in average chemical compositions between D and ID regions are accounted for by an adjustment of volume fractions, the γ-channels and the γʼ-cuboids in both regions had the same composition. Therefore, no effort was made to differentiate between D and ID regions, as far as the chemical compositions of the two phases are concerned.
Results
Elastic properties: The elastic stiffnesses of pseudo-single-crystal ERBO/15 and its variants as obtained by the RUS method at room temperature are presented in Table 4. For comparison, data for ERBO/1 from the literature [41] have been added. Additionally, the elastic compliances sij have been calculated using the relations, which hold for materials with cubic symmetry.
The directional Young’s or elastic modulus E equals the inverse of the longitudinal effect of the elastic compliances. With the direction of interest u = u1e1 + u2e2 + u3e3, where ei describes the basis vectors of a Cartesian reference system and the ui are direction cosine, the E moduli for selected cubic directions are obtained by:
Selected values are presented in Table 4.
The temperature dependence of the elastic stiffnesses is shown in Fig. 6. Between 100 and 673 K, c11, c12 and c44 decrease continuously with increasing temperature by about 8.5%, 6% and 13%, respectively. Temperature coefficients of the cij as determined by linear approximations to experimental data in the temperature range 273–673 K are given in Table 4. In order to describe the temperature dependence of the E moduli in the crystallographic directions < 100 >, < 110 > and < 111 > , the corresponding E<uvw> data were approximated over the entire investigated temperature range by second-order polynomials of the type:
The corresponding parameters and their standard deviations as derived from the covariance matrix of the fully converged fit are given in Table 5. As an example, values for E<100> of ERBO/1 (data from [41]) and the ERBO/15 variants (this work) are shown in Fig. 6d.
Dilatometric results: Thermal expansion results for the four investigated superalloys are presented in Figs. 7 and 8. The experimental strain curves εth = f(T) are all characterized by well reproducible changes in slope at high temperatures. This becomes particularly evident when the thermal expansion coefficients αth = f(T) are plotted as a function of temperature. These curves exhibit a sharp maximum of the thermal expansion coefficient at high temperatures. In Fig. 7, thermal strains and thermal expansion coefficients of the as-cast and fully heat-treated ERBO/15-W are shown.
It can be seen that the αth(T) peak positions of the as-cast and heat-treated materials are close, the peak temperature of the heat-treated material is only 12 K higher than that of the as-cast material. ERBO/1 was investigated in the heat-treated material state. In the case of the ERBO/15 variants, the as-cast material state was analyzed.
ThermoCalc predictions and alloy compositions: ThermoCalc was used to calculate equilibrium phase fractions for all investigated alloys, based on the chemical alloy compositions given in Table 1. These are presented as a function of temperature in Fig. 9. While in ERBO/1 three thermodynamically stable TCP-phases (µ−, σ− and R-phase) are formed at equilibrium, only µ-phase is formed in ERBO15 and its derivatives. With increasing temperature, the TCP and γʼ-phase fractions decrease, while the fraction of the γ-phase increases. In Table 6, the calculated solvus (Tsolvus), solidus (Tsolidus), liquidus (Tliquidus) temperatures together with the γʼ-phase fractions at 873 K and 1323 K taken from the curves presented in Fig. 9 are listed. It becomes apparent that especially the calculated γʼ-solvus temperature for ERBO/1 is about 50 K higher than the solvus temperatures of ERBO/15 and its derivatives. While the calculated solidus temperatures are quite similar, the liquidus temperature of ERBO/1 is the highest of all four alloys. Also, the calculated γʼ-phase fraction fVγʼ at 873 K (74 vol.%) and 1323 K (56 vol.%) is the highest in the case of ERBO/1. When the Mo or W content in ERBO/15 is reduced (balanced by an increase in Ni), the calculated solidus and liquidus temperatures decrease. The reductions result in higher γʼ-phase fractions at 873 K (+ 1 vol.%) but lower γʼ-phase fractions at 1323 K (−3 vol.%).
Calculated and measured phase compositions: The compositions of the γ- and γʼ-phase (cγ and cγʼ ) in the four investigated alloys were measured with 3D APT (ERBO 1) [36] and with TEM-EDX (ERBO 15 and derivatives) [32]. The experimental results for the two phases are presented in Tables 7 (γ-phase) and 8 (γʼ-phase). Tables 7 and 8 also contain ThermoCalc predictions obtained for temperatures at 1143 K (temperature of second precipitation treatment step for all alloys), at 1413 K and 1583 K (ERBO/1; temperature of first precipitation treatment step and homogenization, respectively) and at 1313 K and 1583 K (ERBO/15 variants; temperature of first precipitation treatment step and homogenization, respectively). Since the γ-phase exhibits a smaller volume fraction than the γʼ-phase, changes in its chemical composition are more pronounced. In Figs. 10 and 11, we present chemical compositions for the γ-phase from Table 7 as pie charts. Figure 10 shows experimental data, which were measured in all four heat-treated alloys prior to creep. ThermoCalc predictions obtained for the γ-phases of ERBO/1 (1143, 1413 and 1583 K) and for ERBO/15 (1143, 1313 and 1583 K) are presented in
Fig. 11.
The data presented in Table 7 and Figs. 10 and 11 (γ-phase) and in Table 8 (γʼ-phase, data presented without graphics) show that increasing temperatures result in increasing amounts of Ti, Al and Ta and simultaneously decreasing amounts of Cr, Co, W and Re for ERBO/1 in the γ-phase. As can be seen in the ThermoCalc results presented in Fig. 11, the amount of the base element Ni is increasing with increasing temperature in ERBO/1. In contrast, it decreases with increasing temperature in ERBO/15. The thermodynamic data for the γ- and the γʼ-phases in Table 7 (and Figs. 10 and 11) and Table 8, respectively, further show that the ThermoCalc data for 1143 K (temperature of last precipitation treatment of experimental alloys) and experimentally determined data are not in full agreement but reasonably close to each other for both alloy systems. Only in the case of ERBO/15, the element Mo shows a significantly lower value in the calculation at 1143 K (1.0 at.%) than in the experiment (4.4 at.%).
Discussion
Elastic stiffnesses: As can be seen in Fig. 6a–c, all elastic stiffnesses decrease with increasing temperature. This is mainly a consequence of the anharmonicity of the lattice potential. With increasing temperature, the increasing thermal vibrations lead to larger bond distances, which result in a decrease in bonding interaction and thus in a decrease in elastic stiffnesses. The elastic behavior of ERBO/1 and ERBO/15 is almost identical, whereas the results for the leaner ERBO/15 variants for c11 and c12 fall slightly short. This does not significantly affect the elastic moduli E<100>, which all are very close (Fig. 6d). As can be seen in Table 9, individual alloy elements of SX differ in size, crystal structure, Young’s modulus, electronegativity and melting point [48,49,50,51]. Figure 6d shows that the changes in alloy chemistry considered in the present work do not strongly affect elastic properties. This is in line with the conclusions drawn by Demtröder et al. [41], who showed that even larger variations of alloy compositions than considered in the present work do not strongly affect the elastic properties of SX. The elastic behavior of a single crystal directly reflects the anisotropy of its bonding system. The latter is mainly controlled by type, number and spatial arrangement of nearest-neighbor contacts in the crystal structure. Since the structures of Ni-base SX (including γ/γ'-microstructures) as well as their main chemical compositions (> 62 at.-% Ni, > 11 at.-% Al) differ only slightly, the interactions are dominated by Ni–Ni and Ni–Al contacts, leading to only small variations of the macroscopic elastic stiffnesses [42].
Thermal expansion and γʼ-solvus temperatures: Thermal expansion is associated with a material’s tendency to change its volume with increasing temperature. In a crystal, this is associated with an increasing vibrational energy of the atoms and the non-harmonic shape of the lattice potential. According to the Grüneisen relation, \(\alpha (T)\) is proportional to the heat capacity; thus, the thermal strain \(\varepsilon (T)\) can be described by an integrated form of the Einstein model [52, 53]:
\(\varepsilon_{0}\) represents the initial strain at 0 K, \(\alpha_{h}\) denotes the high-temperature limit of the thermal expansion coefficient, and \(\theta_{E}\) is the equivalent of the Einstein temperature. The first derivative with respect to temperature yields the thermal expansion coefficient:
The Einstein model usually provides a good approximation of heat capacity and thermal expansion at temperatures above about \(\theta_{E}\)/2. In the case of the superalloys investigated in this work, the Einstein approach describes well the observed thermal strains and thermal expansion coefficients up to about 800 K with \(\theta_{E}\) varying between 396 and 412 K (Fig. 12a, c). However, at higher temperatures significant differences occur as expressed in Fig. 12a by the thermal excess strain, which represents the difference between the experimental thermal strain \(\varepsilon_{\exp }\)(T) (black curve) and the extrapolated strain \(\varepsilon_{fit}\)(T) (red curve, Eq. 3) determined by fitting an Einstein model to \(\varepsilon_{\exp }\)(T) below 800 K. The experimental curve further undergoes a change of slope, which can be better appreciated considering its first derivative \(\alpha_{\exp }\)(T), black curve in Fig. 12c. In Fig. 12b, \(\Delta \varepsilon^{*}\)(T) (black curve) is presented together with the evolution of the γ-volume fraction \(f_{\gamma }\)(T) (red curve) as predicted by ThermoCalc. It can be clearly seen that both curves show similar trends, which is even more evident for their first derivatives (Fig. 12d). This strongly suggests that the temperatures, where changes of slope of the \(\varepsilon_{th}\)-curves are detected, i.e., where the \(\alpha_{th}\)(T)-curves show a sharp peak, represent γʼ-solvus temperatures. Similar effects have been reported for ternary Ni–Fe–Al alloys [54], CMSX-2 [55] and Co-based alloys [56, 57].
Rationalization of the correlation between thermal expansion and γ-volume fraction. a Experimental and fitted thermal strains, \(\varepsilon_{\exp }\)(T) and \(\varepsilon_{fit}\)(T). b Evolution of the excess strain \(\Delta \varepsilon^{*}\)(T) and γ-volume fraction \(f_{\gamma }\)(T). c First derivatives of experimental and fitted thermal strains (thermal expansion coefficients, \(\alpha_{\exp }\)(T) and \(\alpha_{fit}\)(T)). d First derivatives of \(\varepsilon_{\exp }\)(T)-(experimental data) and \(f_{\gamma }\)(T)-curves. For details, see text
Figure 13 schematically illustrates how the experimentally observed thermal expansions can be rationalized. In a first-order approximation, one can assume that the thermal expansions of the two isolated phases each follow an Einstein model (Eq. 5). Different model parameters result in the fact that at high temperatures, the γ-phase (green curve) reaches significantly higher values than the γʼ-phase (blue curve). The red line schematically illustrates the experimental data for a superalloy, which contains both phases (Fig. 3). The thermal expansion of the γʼ-phase (high initial γʼ volume fractions close to 70%) dominates for T < 800 K. Starting at about 800 K, the gradual dissolution of the γʼ-precipitates and the corresponding increase in the volume fraction of the γ-phase (Fig. 12b) are associated with an adjustment of the chemical equilibrium compositions of the two phases. The resulting changes in unit cell dimensions and γ/γʼ-volume fraction ratios cause the sharp peak in the experimentally measured thermal expansion close to Tsolvus (Figs. 7, 8, 12c and d). About 50% of the excess strain Δε* shown in Fig. 12a can be rationalized by the decreasing effect of the lattice misfit (estimate for ERBO15 and its variants: 5 × 10–3), which provides additional contributions to the thermal strain. The remaining part of Δε* is probably related to changes of the unit cell dimensions of both phases related to an increase in configurational entropy. Additionally, the volume fraction of the γ-phase, which shows a higher coefficient of thermal expansion than the γʼ-phase, increases with increasing temperature. This is in line with experimental data from the literature on the thermal expansion of isolated γ- and γʼ-phases of CMSX-4 [58] and on a small step-like increase in heat capacity around about 870 K in CMSX-4 reported in [59].
With increasing temperature the vacancy density increases, as was reported for Al in the seminal work of Simmons and Balluffi [60]. However, this effect is usually very small and increases exponentially up to the melting temperature of the material. It is not related to the sharp peak observed in the experimental αth(T)-curves. Similar effects have been reported for example for the order/disorder transformations in CuAu [61] and Ag3Mg [62].
The dilatometric results of Fig. 8 and the CALPHAD predictions of Fig. 9 are combined in Fig. 14. The dilatometric curves exhibit a sharp maximum of thermal expansion at high temperatures, which for ERBO/1-C (1557 K) coincides with the γʼ-solvus temperature (1555 K) predicted by ThermoCalc (Fig. 14a). However, for all three as-cast ERBO/15 variants, the αth(T)-maxima are observed at temperatures, which are about 40 K higher than the γʼ-solvus temperatures predicted by ThermoCalc (Fig. 14b–d). In Table 10, the peak temperatures from Figs. 7, 8 and 14 of all four investigated alloys are shown.
In Fig. 15, we compare our ERBO/1 thermal expansion data (presented in red) with results which were published in the literature. The elastic ERBO/1 data we have used so far represent true αth data (red solid line), which were obtained as described in the experimental section of this work. In Fig. 15, we show these data together with mean αth data, which were calculated using 295 K as reference temperature according to:

taken from the present work (ERBO/1-C) and literature. Our data are shown in red, true expansion coefficients full red line, mean expansion coefficient dashed red line). Ni (full circles) data were taken from Sung et al. [63]; Ni3Ti (empty circles) were taken from the work of Karunaratne et al. [68]; CMSX-4 data (dashed black line): Quested et al. [59]; Isolated γ (empty squares) and isolated γʼ (full squares) of CMSX-4 taken from Siebörger et al. [58]; SX with Mo (empty triangles) from Morrow et al. [71]
Thermal expansion coefficients vs. temperature data, which were
This is a quantity, which is frequently used in the literature. As can be seen in Fig. 15, the true αth(T) data show the sharp peak, which we exploit for the determination of γʼ-solvus in the present work, while this feature is largely smoothed out by averaging in the case of \(\alpha_{{\text{th - MEAN}}} (T)\). Figure 15 presents digitalized data from different sources [58, 59, 63,64,65,66,67,68,69,70,71]. All authors performed their experiments using heating rates between 2 K/min and 5 K/min. Data for Ni (full circles) were reproduced from the work of Sung et al. [63], who have created a regression line from various previous data [64,65,66,67] and the data for Ni3Ti (empty circles) were reproduced from the work of Karunaratne et al. [68] (who refer to previous publications [65, 69, 70]). It is interesting to compare these data with dilatometric results for the γ-phase (empty squares) and for the γʼ-phase data (full squares), which were isolated from CMSX-4 by Siebörger et al. [58]. Their data represent true thermal expansion; however, the isolated phases cannot adjust their chemical composition to γ/γʼ-equilibrium. Thus, no peak in thermal expansion like that observed in our work appears. Morrow et al. [71] investigated the influence of Mo additions to a Ni-base superalloy with γ/γʼ-microstructure and showed that increasing Mo levels and Al-levels result in a small decrease in thermal expansion coefficients. In Fig. 15, we reproduce their data for a Ni-based alloy with 3.5% Mo (empty triangles). Finally, we add the recently published CMSX-4 data set from Quested et al. [59] (thick dashed line). The comparison reveals that while there is some scatter, all data are reasonably close when we compare them to our mean thermal expansion coefficient. Note that our mean thermal expansion data and the Quested et al. [59] are in excellent agreement. Our true expansion data, however, deviate significantly toward higher values and show the sharp peak, which allows to determine the γʼ-solvus temperature.
Our true thermal expansion data show a pronounced sharp peak (highlighted by an arrow) at high temperatures where the thermal expansion coefficient drops by almost 50%. The data presented in Figs. 7, 8, 9, 13 and 14 clearly suggest that this drop is associated with the γʼ-solvus temperature. For ERBO/1 (CMSX-4 type), the drop occurs at a temperature, which is very close to the γʼ-solvus temperature as predicted by ThermoCalc. In case of the three ERBO/15-type alloys, the thermal expansion drop occurs at temperatures, which are 40 K above the predicted γʼ-solvus temperatures. There is a better agreement between measured (dilatometry) and calculated (ThermoCalc) γ’-solvus temperatures for ERBO/1 than for ERBO/15 and its variants (Tables 7 and 8, Figs. 10, 11). This is in line with the finding that the experimentally determined alloy compositions for ERBO/1 (3D-APT, [36], standard material of CMSX-4 type) are in better agreement with the corresponding ThermoCalc predictions than in the case of the experimental ERBO/15 alloys (experimental data: TEM–EDX, [32]). The discrepancy between the γʼ-solvus temperatures determined experimentally and the ThermoCalc predictions for the ERBO/15 alloys as well as the differences in phase compositions measured in TEM and predicted by ThermoCalc suggest that the ThermoCalc data base needs to be optimized for the new compositional range. The experimental results and the ThermoCalc predictions both suggest that reducing Mo or W levels has no significant effects on the γʼ-solvus temperatures.
The focus of the present work was on determining γʼ-solvus temperatures using true thermal expansion measurements. In addition, we report elastic coefficients for four Ni-base single-crystal superalloys, which are useful for engineering design in stress temperature regimes where elasticity governs mechanical material behavior and for estimating thermal stresses associated with thermal fatigue loading. Our results are not directly applicable to assess creep properties. However, γʼ-solvus temperatures are a measure for the stability of γʼ-particles, which provide creep strength. Our results is therefore indirectly related to the creep behavior of single-crystal Ni-base superalloys.
Summary and conclusions
The present work investigates the influence of alloy chemistry on elastic properties and on γʼ-solvus temperatures in Ni-base single-crystal superalloys. Results obtained for a CMSX-4 type of alloy (referred to as ERBO/1 with Re) are compared with three experimental Re-free alloys referred to as ERBO/15, ERBO/15-W (less W than ERBO 15) and ERBO/15-Mo (less Mo than ERBO 15). From the results obtained in the present work, the following conclusions can be drawn:
-
(1)
The elastic properties of ERBO/15 and its two leaner derivatives do not strongly deviate from the ERBO/1 data, which were recently reported by Demtröder et al. [41]. Fit equations, which describe the temperature dependencies of the elastic moduli E<100>, E<110> and E<111>, are reported.
-
(2)
Decreasing W and Mo levels in ERBO/15 result in a small decrease in elastic stiffness (c11, c12). This effect correlates with the electronegativity rather than with the atomic size. It does not strongly affect the values of the macroscopic elastic moduli.
-
(3)
The thermal expansion behavior of superalloy single crystals does not strongly depend on whether the as-cast or fully heat-treated conditions are considered.
-
(4)
A comparison between thermodynamic CALPHAD calculations and experimentally observed temperature dependencies of the thermal expansion coefficients suggests that the transition from a low-temperature material state with a high γʼ-volume fraction to a single-phase γ-material at high temperatures governs the observed behavior.
-
(5)
A sharp peak in the true thermal expansion coefficient vs. temperature curves indicates the γʼ-solvus temperature. The experimental peak positions are in excellent agreement with ThermoCalc predictions for ERBO/1. There is a difference of the order of 40 K in case of ERBO/15 and its two leaner derivatives. This is most probably related to the quality of thermodynamic data available for the new alloy compositions, which can be concluded from the fact that experimental measurements of phase compositions also show some deviation from CALPHAD predictions.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
References
Durand-Charre M (1998) The Microstructure of superalloys. Taylor and Francis, Amsterdam
Pollock TM, Tin S (2006) Nickel-based superalloys for advanced turbine engines: Chemistry, microstructure and properties. J Propuls Power 22:361–374
Reed RC (2008) The superalloys: fundamentals and applications. Cambridge University Press, England
Bürgel R, Maier HJ, Niendorf T (2011) Handbuch der Hochtemperatur-Werkstofftechnik. Vieweg Verlag, Wiesbaden
Völkl R, Glatzel U, Feller-Kniepmeier M (1998) Measurement of lattice misfit in the single crystal nickel based superalloys CMSX-4, SRR 99 and SC 16 by convergent beam electron diffraction. Acta Mater 46:4395–4404
Mughrabi H (2014) The importance of sign and magnitude of γ/γ′ lattice misfit in superalloys—with special reference to the new γ′-hardened cobalt-base superalloys. Acta Mater 81:21–29
Pineau A (1976) Influence of uniaxial stress on morphology of coherent precipitates during coarsening – elastic energy considerations. Acta Met 24:559–564
Kuhn HA, Biermann H, Ungar T, Mughrabi H (1991) An X-ray study of creep-deformation induced changes of the lattice mismatch in the γ’ hardened monocrystalline nickel-base superalloy SRR-99. Acta Met Mat 39:2783–2794
Pollock TM, Argon A (1992) Creep resistance of CMSX-3 nickel-base superalloy single crystals. Acta Met Mat 40:1–30
Field RD, Pollock TM, Murphy WH (1992) The development of γ/γ’ interfacial dislocation networks during creep in Ni-base superalloys. In: Superalloys 1992, Antolovich SD et al (eds), TMS, Pennsylvania, pp 557–566
Socrate S, Parks DM (1993) Numerical determination of the elastic driving force for directional coarsening in Ni-superalloys. Acta Met Mat 41:2185–2209
Keller RR, Maier HJ, Mughrabi H (1993) Characterization of interfacial dislocation networks in a creep-deformed nickel-base superalloy. Scripta Met Mat 28:23–28
Eggeler G, Dlouhy A (1997) On the formation of <010>-dislocations in the γ’-phase of superalloy single crystals during high temperature and low stress creep. Acta Mater 45:4251–4262
Probst-Hein M, Dlouhy A, Eggeler G (1999) Interface dislocations in superalloy single crystals. Acta Mater 47:2497–2510
Matan N, Cox DC, Rae CMF, Reed RC (1999) On the kinetics of rafting in CMSx-4 superalloy single crystals. Acta Mater 47:2031–2045
Nembach E (1997) Particle Strengthening of Metals and Alloys. John Wiley and Sons, New York
Chandran M, Sondhi SK (2011) First-principle calculation of APB energy in Ni-based binary and ternary alloys. Modelling Simul Mater Sci Eng 19:025008–025015
Vamsi KV, Karthikeyan S (2012) Effect of off-stoichiometry and ternary additions on planar fault energies in Ni3Al. In: Superalloys 2012, Huron ES et al (eds). TMS, Pennsylvania, pp 521–530
Long HB, Liu YN, Kong DL, Wei H, Chen YH, Mao SC (2017) Shearing mechanisms of stacking fault and anti-phase boundary forming dislocation pairs in the γ’-phase of Ni-base superalloys. J Alloys Compounds 724:287–295
Kumar K, Sankarasubramanian R, Waghmare UV (2018) Tuning planar fault energies of Ni3Al with substitutional alloying: First-principles description for guiding rational alloy design. Scripta Mater 142:74–78
Gleiter H, Hornbogen E (1965) Aushärtung durch kohärente geordnete Teilchen. Acta Metall 13:576–578
Vorontsov VA, Shen C, Wang Y, Dye D, Rae CMF (2010) Shearing of γ’-precipitates by <112> dislocation ribbons in Ni-base superalloys: A phase field approach. Acta Mater 58:4110–4119
Eggeler Y, Titus M, Suzuki A, Pollock T (2014) Creep deformation-induced antiphase boundaries in L12-containing single-crystal cobalt-base superalloys. Acta Mater 77:352–359
Sato A, Moverare JJ, Hasselquist M, Reed RC (2012) On the mechanical behavior of a new single-crystal superalloy for industrial gas turbine applications. Met Mat Trans A 43:2302–2315
Qiu CL, Andrews P (2013) On the formation of irregular-shaped γ’ and serrated grain boundaries in a nickel-based superalloy during continuous cooling. Mat Char 76:28–34
Gilles R, Mukherjeed D, Eckerlebe H, Karge L, Staron P, Strunz P, Lippmann Th (2014) Investigations of early stage precipitation in a tungsten-rich nickel-base superalloy using SAXS and SANS. J Alloys Comp 612:90–97
Raza SS, Ahmad T, Manzoor MU, Kamran M, Ahmad R (2017) Analysis of metallurgical aspects and their role in processing and performance of superalloys. J Fundamental Appl Sci 9:485–498
Bassini E, Marchese G, Cattano G, Lombardi M, Biamino S, Ugues D, Vallilo G, Picque B (2017) Influence of solutioning on microstructure and hardness of hot isostatically pressed Astroloy. J Alloys Compounds 723:1082–1090
D’Souza N, Li W, Argyrakis C, West GD, Slater CD (2019) On the evolution of primary γ’ precipitates during high temperature and high strain rate deformation and subsequent heat treatment in the Ni-based superalloy RR 1000. Met Mat Trans A 50:4205–4222
Pollock TM, Van der Ven A (2019) The evolving landscape for alloy design. MRS Bull 44:238–246
Kumari G, Boehlert C, Sankaran S, Sundaraman M (2020) The effect of solutionizing temperature on the microstructure of Allvac 718 Plus. J Mat Eng Perf. https://doi.org/10.1007/s11665-020-04687
Horst OM, Adler D, Git P, Wang H, Streitberger J, Holtkamp M, Jöns N, Singer RF, Körner C, Eggeler G (2020) Exploring the fundamentals of Ni-based superalloy single crystal (SX) alloy design: Chemical composition vs. microstructure. Mater Des 195:108976
Horst OM, Ibrahimkhel S, Streitberger J, Wochmjakow N, Git P, Scholz F, Thome P, Singer RF, Körner C, Frenzel J, Eggeler G (2020) On the influence of alloy composition on creep behavior of Ni-based single-crystal superalloys (SXs). In: Superalloys 2020, Tin S et al (eds). TMS, Pennsylvania, pp 60–70
Rettig R, Matuszewski K, Müller A, Helmer HE, Ritter NC, Singer RF (2016) Development of a low‐density rhenium‐free single crystal nickel‐based superalloy by application of numerical multi‐criteria optimization using thermodynamic calculations. In: Superalloys 2016, Hardy MC et al (eds). TMS, Pennsylvania, pp 35–44
Andersson J-O, Helander T, Höglund L, Shi P, Sundman B (2002) THERMO-CALC & DICTRA, Computational tools for materials science. Calphad 26:273–312
Parsa AB, Wollgramm P, Buck H, Somsen C, Kostka A, Povstugar I, Choi P, Raabe D, Dlouhy A, Müller J, Spiecker E, Demtröder K, Schreuer J, Neuking K, Eggeler G (2015) Advanced scale bridging microstructure analysis of single crystal Ni-based superalloys. Adv Eng Mat 17:216–230
Wu X, Wollgramm P, Somsen C, Dlouhy A, Kostka A, Eggeler G (2016) Double minimum creep of single crystal Ni-base superalloys. Acta Mater 112:242–260
Every AG, Sachse W (2001) Handbook of elastic properties of solids, liquids, and gases. Academic Press, London
Leisure RG, Willis FA (1997) Resonant ultrasound spectroscopy. J phy condensed matter. 9(28):6001
Wollgramm P, Bürger D, Parsa AB, Neuking K, Eggeler G (2016) The effect of stress, temperature and loading direction on the creep behaviour of Ni-base single crystal superalloy miniature tensile specimens. Mater High Temp 33:346–360
Demtröder K, Eggeler G, Schreuer J (2015) Influence of microstructure on macroscopic elastic properties and thermal expansion of nickel-base superalloys ERBO/1 and LEK94. Materialwiss Werkstofftech 46:563–576
Swalin RA (1972) Thermodynamics of solids. Wiley, New York
Gaskell DR (2003) Introduction to the thermodynamics of materials. Taylor and Francis, New York
Porter DA, Easterling KE, Sherif MY (2009) Phase transformations in metals and alloys. CRC Press, Boca Raton
Kaufman L, Bernstein H (1970) Computer calculation of phase diagrams with special reference to refractory metals. Academic Press Inc, New York
Lukas HL, Fries SG, Sundman B (2007) Computational thermodynamics: The calphad method. Cambridge University Press, Cambridge
Yardley V, Povstugar I, Choi PP, Raabe D, Parsa AB, Kostka A, Somsen C, Dlouhy A, Neuking K, George EP, Eggeler G (2016) On local phase equilibria and the appearance of nanoparticles in the microstructure of single-crystal Ni-base superalloys. Adv Eng Mater 18:1556–1567
Slater JC (1964) Atomic radii in crystals. J Chem Phys 41:3199–3204
Pauling L (1967) The chemical bond. Cornell University Press, New York
James AM, Lord MP (1992) Macmillan’s chemical and physical data. Macmillan, London
Davis JR (1998) Metals handbook. ASM International, Ohio
Schulze GER (1974) Metallphysik, 2nd edn. Springer Verlag, Berlin
Weißmantel C, Hamann C (1979) Grundlagen der Festkörperphysik. Springer Verlag, Berlin
Cahn RW, Siemers PA, Geiger JE, Bardhan P (1987) The order-disorder transformation in Ni3Al and Ni3Al-Fe Alloys – I. Determination of the transition temperatures and their relation to ductility, Acta Met 35:2737–2751
Grosdidier T, Hazotte A, Simon A (1998) Precipitation and dissolution process in γ/γ’ single crystal nickel-based superalloys. Mater Sci Eng A 256:183–196
Jackson RW, Titus MS, Begley MR, Pollock TM (2016) Thermal expansion behavior of new Co-based alloys and implications for coatings. Surf & Coat Techn 289:61–68
Volz N, Zenk CH, Cherukuri R, Kalfhaus T, Weiser M, Makineni SK, Betzing C, Lenz M, Gault B, Fries SG, Schreuer J, Vassen R, Virtanen S, Raabe D, Spiecker E, Neumeier S, Göken M (2018) Thermophysical and mechanical properties of advanced single crystalline Co-base superalloys. Met Mat Trans A 49A:4099–4109
Siebörger D, Brehm H, Wunderlich F, Möller D, Glatzel U (2001) Temperature dependence of lattice parameter, misfit and thermal expansion coefficient of matrix, γ’ phase and superalloy. Z Metallkde 92:58–61
Quested PN, Brooks RF, Chapman L, Morrell R, Youssef Y, Mills KC (2009) Measurement and estimation of thermophysical properties of nickel based superalloys. Mater Sci and Techn 25:154–162
Simmons RO, Balluffi RW (1960) Measurements of equilibrium vacancy concentrations in aluminum. Phys Rev 117:52–61
Hämäläinen M, Laine E, Tarna T (1969) Thermal expansion of the Ag3Mg Alloy in the ordered and disordered states. J Appl Cryst 2:95–101
Anraku T, Sakaihara I, Hoshikawa T, Taniwaki M (2009) Phase transition and thermal expansion behavior in AuCu alloy. Mat Trans 50:683–688
Sung PK, Poirier DR (1998) Estimation of densities and coefficients of thermal expansion of solid Ni-base superalloys. Mater Sci Eng A 245:135–141
Goldsmith TE, Waterman TE, Hirschhorn HJ (1961) Handbook of Thermophysical Properties of Solid Materials. Macmillan, New York
Touloukian YS, Kirby RK, Taylor RE, Desai PD (1975) Thermophysical properties of matter - the TPRC data series. Purdue University, Indiana, Thermal expansion metallic elements and alloys
Drotning WD (1982) Thermal Expansion of Nickel to 2300 K. In: Thermal Expansion 7, Larsen DC (ed). Springer, Boston
Brandes EA, Brook GB (1992) Smithells Metals Reference Book. Butterworth-Heinemann, Oxford
Karunaratne MSA, Kyaw S, Jones A, Morrell R, Thomson RC (2016) Modelling the coefficient of thermal expansion in Ni-based superalloys and bond coatings. J Mater Sci 51:4213–4226
Taylor A, Floyd RW (1953) The constitution of nickel-rich alloys of the nickel-titanium-aluminum system. J Inst Met 81:25
Arbuzov MP, Zelenkov IA (1964) Thermal expansion of certain transition metals and alloys on their base. Phys Met Metallogr 18:311–312
Morrow H, Sponseller DL, Semchyshen M (1975) The effects of molybdenum and aluminum on the thermal expansion coefficients of nickel-base alloys. Met Trans A 6A:477–485
Acknowledgement
This work was funded by the German research association (Deutsche Forschungsgemeinschaft, DFG) in the framework of the collaborative research center SFB/TR 103. The authors appreciate funding through projects A1 (OMH, EG), B1 (PG, CK) and Z01 (PG, CK). The authors would like to acknowledge fruitful discussions with Dr. Suzana G. Fries (Computational Thermodynamics: CALPHAD, Scientific Guest, Ruhr-University Bochum) and the performance of field emission electron microprobe measurements by Dr. Niels Jöns and Sabine Weisel (Department of Geology, Mineralogy and Geophysics, Ruhr-University Bochum).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Our work is funded by through the collaborative research center SFB/TR 103 (Fundamentals for a new superalloy technology). We have no direct commercial interest. No experiments involving human tissue were carried out.
Additional information
Handling Editor: P. Nash.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horst, O.M., Schmitz, D., Schreuer, J. et al. Thermoelastic properties and γ’-solvus temperatures of single-crystal Ni-base superalloys. J Mater Sci 56, 7637–7658 (2021). https://doi.org/10.1007/s10853-020-05628-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05628-w