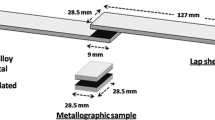
Abstract
The transient liquid phase (TLP) bonding of Ti-6Al-4V alloy to a Mg-AZ31 alloy was performed using an electrodeposited Ni coating containing a dispersion of Ni and Cu nanoparticles. Bond formation was attributed to two mechanisms; first, solid-state diffusion of Ni and Mg, followed by liquid eutectic formation at the Mg-AZ31 interface. Second, the solid-state diffusion of Ni and Ti at the Ti-6Al-4V interface resulted in a metallurgical joint. The joint interface was characterized by scanning electron microscopy, energy dispersive X-ray spectroscopy, and X-ray diffraction analysis. Microhardness and shear strength tests were used to investigate the mechanical properties of the bonds. The use of Cu nanoparticles as a dispersion produced the maximum joint shear strength of 69 MPa. This shear strength value corresponded to a 15 % enhancement in joint strength compared to TLP bonds made without the use of nanoparticles dispersion.












Similar content being viewed by others
References
Sakiyama T, Murayama G, Kenji S et al (2013) Dissimilar metal joining technologies for steel sheet and aluminum alloy sheet in auto body. Nippon Steel Tech Rep 103:91–98
AlHazaa A, Khan TI, Haq I (2010) Transient liquid phase (TLP) bonding of Al7075 to Ti–6Al–4V alloy. Mater Charact 61:312–317
Elthalabawy W, Khan TI (2011) Liquid phase bonding of 316L stainless steel to AZ31 magnesium alloy. J Mater Sci Technol 27:22–28
Chang C-T, Shiue R-K (2006) Infrared brazing of Ti-6Al-4V using the Ti-15Cu-15Ni braze alloy. J Mater Sci 41:2145–2150. doi:10.1007/s10853-006-5242-7
Jin YJ, Khan TI (2012) Effect of bonding time on microstructure and mechanical properties of transient liquid phase bonded magnesium AZ31 alloy. Mater Des 38:32–37. doi:10.1016/j.matdes.2012.01.039
Atieh AM, Khan TI (2013) Effect of process parameters on semi-solid TLP bonding of Ti–6Al–4V to Mg–AZ31. J Mater Sci 48:6737–6745. doi:10.1007/s10853-013-7475-6
Atieh AM, Khan TI (2014) Transient liquid phase (TLP) brazing of Mg-AZ31 and Ti-6Al-4V using Ni and Cu sandwich foils. Sci Technol Weld Join. doi:10.1179/1362171814Y.0000000196
Sun D, Gu X, Liu W (2005) Transient liquid phase bonding of magnesium alloy (Mg-3Al-1Zn) using aluminum interlayer. Mater Sci Eng A 391:29–33
Saha RK, Khan TI (2009) Microstructural developments in TLP bonds using thin interlayers based on Ni–B coatings. Mater Charact 60:1001–1007. doi:10.1016/j.matchar.2009.04.002
Cook G, Sorensen C (2011) Overview of transient liquid phase and partial transient liquid phase bonding. J Mater Sci 46:5305–5323. doi:10.1007/s10853-011-5561-1
Zhou Y, Gale WF, North TH (1995) Modelling of transient liquid phase bonding. Int Mater Rev 40:181–196. doi:10.1179/095066095790151160
Zhang G, Zhang J, Pei Y et al (2008) Joining of Al2O3p/Al composites by transient liquid phase (TLP) bonding and a novel process of active-transient liquid phase (A-TLP) bonding. Mater Sci Eng A 488:146–156. doi:10.1016/j.msea.2007.11.084
Cooke KO (2012) A study of the effect of nanosized particles on transient liquid phase diffusion bonding Al6061 metal-matrix composite (MMC) Using Ni/Al2O3 nanocomposite interlayer. Metall Mater Trans B 43:627–634. doi:10.1007/s11663-012-9643-5
Cooke KO, Khan TI, Oliver GD (2012) Transient liquid phase diffusion bonding Al-6061 using nano-dispersed Ni coatings. Mater Des 33:469–475. doi:10.1016/j.matdes.2011.04.051
Tiwari SK (2010) Nickel Nanoparticles-Assisted Diffusion Brazing of Stainless Steel 316 for Microfluidic Applications. Oregon State University
Smith WF (1993) Structure and properties of engineering alloys. McGraw-Hill Inc, New York
Chang CT, Shiue RK (2005) Infrared brazing Ti–6Al–4V and Mo using the Ti–15Cu–15Ni braze alloy. Int J Refract Met Hard Mater 23:161–170. doi:10.1016/j.ijrmhm.2005.01.002
Kulekci MK (2007) Magnesium and its alloys applications in automotive industry. Int J Adv Manuf Technol 39:851–865. doi:10.1007/s00170-007-1279-2
Friedrich HE, Mordike BL (2006) Magnesium Technology Metallurgy, Design Data, and Applications. doi: 10.1007/3-540-30812-1
Elthalabawy W (2010) Diffusion Bonding behavior and characterization of joints made between 316L stainless steel alloy and AZ31 magnesium alloy. University of Calgary
Vušanovi I, Voronjec D, Krane MJM (2001) Microsegregation phenomena in Al–Cu–Mg alloy with considering of diffusion phenomena in primary phase. Facta Univ 1:965–980
Castro T, Reifenberger R, Choi E, Andres RP (1990) Size dependence melting point of individual nanometer-sized metallic clusters. Phys Rev B 42:8548–8557
Jiang H, Moon K, Dong H et al (2006) Size-dependent melting properties of tin nanoparticles. Chem Phys Lett 429:492–496
Ryan EJ (1988) Titanium–copper–nickel braze filler metal and method of brazing. US Patent US4725509A, 18 Feb 1986
Tiwari SK, Paul BK (2010) Comparison of nickel nanoparticle-assisted diffusion brazing of stainless steel 316 to conventional diffusion brazing and bonding processes. J Manuf Sci Eng 132(3):030902-1–030902–5
Sayyedain SS, Salimijazi HR, Toroghinejad MR, Karimzadeh F (2014) Microstructure and mechanical properties of transient liquid phase bonding of / Al nanocomposite using copper interlayer. Mater Des 53:275–282. doi:10.1016/j.matdes.2013.06.074
Gupta KP (2004) The Cu–Mg–Ni (Copper–Magnesium–Nickel) System. J Phase Equilibria Diffus 25:471–478. doi:10.1361/15477030420214
Miettinen J (2008) Thermodynamic description of Cu–Mg–Ni and Cu–Mg–Zn systems. CALPHAD Comput Coupling Phase Diagrams Thermochem / ASM Alloy Phase Diagrams Center 32:389–398
Lee J, Mori H, Tanaka T, Penttilä K (2005) Phase diagrams of nanometer-sized particles in binary systems. JOM 57:56–59. doi:10.1007/s11837-005-0235-6
Lee J-G, Mori H, Yasuda H (2005) In situ high-resolution electron microscope observation of phase change in nanometer-sized alloy particles. J Mater Res 20:1708–1721. doi:10.1557/JMR.2005.0223
Schamp C, Jesser W (2006) Two-phase equilibrium in individual nanoparticles of Bi–Sn. Metall Mater Trans A 37:1825–1829
Tuah-Poku I, Dollar M, Massalski T (1988) A study of the transient liquid phase bonding process applied to a Ag/Cu/Ag sandwich joint. Metall Trans A 19:675–686
Tjong SC (2007) Novel nanoparticle-reinforced metal matrix composites with enhanced mechanical properties. Adv Eng Mater 9:639–652. doi:10.1002/adem.200700106
Sun DQ, Lang B, Sun DX, Li JB (2007) Microstructure and mechanical properties of resistance spot welded magnesium alloy joints. Mater Sci Eng A 460–461:494–498
Tang Y, Zhao X, Jiang K et al (2010) The influences of duty cycle on the bonding strength of AZ31B magnesium alloy by microarc oxidation treatment. Surf Coat Technol 205:1789–1792
Okamoto H (2007) Mg–Ni (Magnesium–Nickel) supplemental literature review: section III. J Phase Equilibria Diffus 28:303. doi:10.1007/s11669-007-9058-1
Bormann R, Zöltzer K (1992) Determination of the thermodynamic functions and calculation of phase diagrams for metastable phases. Phys Status Solidi A / ASM Alloy Phase Diagrams Center 131:691–705
Turchanin MA, Agraval PG, Abdulov AR (2008) Thermodynamic assessment of the Cu–Ti–Zr system. I. Cu–Ti system. Powder Metall Met Ceram / ASM Alloy Phase Diagrams Center 47:344–360
Miettinen J (2005) Thermodynamic description of the Cu–Al–Ni system at the Cu–Ni side, Cu–Ni phase diagram. CALPHAD Comput Coupling Phase Diagrams Thermochem / ASM Alloy Phase Diagrams Center 29:40–48
Prima SB (1993) Aluminium–magnesium–nickel, ternary alloys. VCH/ASM Alloy Phase Diagrams Cent 6:454–459
Gupta KP (2002) The Cu–Ni–Ti (copper–nickel–titanium) system. J Phase Equilibria / ASM Alloy Phase Diagrams Center 23:541–547
Buhler T, Fries SG, Spencer PJ, Lukas HL (1998) A thermodynamic assessment of the Al–Cu–Mg ternary system. J Phase Equilibria / ASM Alloy Phase Diagrams Center 19:317–333
Acknowledgements
The authors would like to acknowledge The German Jordanian University (GJU), and NSERC Canada for the financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atieh, A.M., Khan, T.I. Application of Ni and Cu nanoparticles in transient liquid phase (TLP) bonding of Ti-6Al-4V and Mg-AZ31 alloys. J Mater Sci 49, 7648–7658 (2014). https://doi.org/10.1007/s10853-014-8473-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8473-z