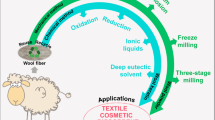
Abstract
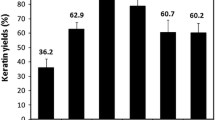
For the first time, pure protein fibers with good mechanical properties were obtained from waste wool after effective de-crosslinking and disentanglement of the highly crosslinked keratin. Every year, more than 1.6 billion pounds of wool keratin-based materials were discarded. Effort has been devoted to convert the keratin resources into high value-added products, especially fibers. In 1940s, pure keratin fibers had been developed from feathers. However, after trying the method, we found the results were not repeatable and did not find any other successful repetition. So far, no effective dissolution and spinning methods have been developed to obtain pure keratin fibers with potential for real applications. In this research, keratin with preserved backbones after de-crosslinking was obtained. Subsequently, surfactant endowed the keratin with satisfactory stretchability for fiber spinning. Fibers of 20 μm with good tensile strength were successfully developed. The technology could be applied onto other highly crosslinked proteins, including feather keratin from poultry industry, sorghum protein and soy protein in bioenergy co-products for production of various industrial products.










Similar content being viewed by others
References
Shishoo R (2012) Technological advances and future challenges. The global textile and clothing industry. Elsevier, Amsterdam, pp 8–28
Rauschendorfer LM (2013), Global Development of Fiber Production. http://www.chemistryviews.org/details/news/4723361/Global_Development_of_Fiber_Production.html. Accessed 16 June, 2013
Johnson J, MacDonald S, Meyer L, Norrington B and Skelly C (2014), The World and United States Cotton Outlook,http://www.usda.gov/oce/forum/2014_Speeches/Cotton.pdf. Accessed 2 May, 2014
Chen HL, Burns LD (2006) Environmental analysis of textile products. Cloth Textiles Res J 24:248–261
U.S. Environmental Protection Agency (2013). http://www.epa.gov/osw/conserve/materials/textiles.htm. Accessed 26 Feburary, 2014
Food and Agricultural Organizations of the United Nations (2013). http://faostat.fao.org/site/569/DesktopDefault.aspx?PageID=569#ancor. Accessed 26 Feburary, 2014
Poole AJ, Church JS, Huson MG (2008) Environmentally Sustainable Fibers from Regenerated Protein. Biomacromolecules 10:1–8
Wen G, Naik R, Cookson P, Smith S, Liu X, Wang X (2010) Wool powders used as sorbents to remove Co2+ ions from aqueous solution. Powder Technol 197:235–240
Rajkhowa R, Zhou Q, Tsuzuki T, Morton DA, Wang X (2012) Ultrafine wool powders and their bulk properties. Powder Technol 224:183–188
Yang Y, Reddy N (2013) Potential of using plant proteins and chicken feathers for cotton warp sizing. Cellulose 20(4):2163–2174
Huda S, Yang Y (2009) Feather Fiber Reinforced Light-Weight Composites with Good Acoustic Properties. J Polym Environ 17:131–142
Hu C, Reddy N, Yan K, Yang Y (2011) Acetylation of chicken feathers for thermoplastic applications. J Agri Food Chem 59:10517–10523
Sun P, Liu ZT, Liu ZW (2009) Chemically Modified Chicken Feather as Sorbent for Removing Toxic Chromium (VI) Ions. Ind Eng Chem Res 48:6882–6889
Yang Y, Reddy N (2013) Potential of using plant proteins and chicken feathers for cotton warp sizing. Cellulose 20:2163–2174
Harris M, Brown AE (1947) Natural and Synthetic Protein Fibers. Text Res J 17:323–330
Evans RL, Shore B (1948) U.S. Patent. 2 434 688, Regenerated Keratin
Wormell RL, Happey F (1949) Regenerated Keratin Fibres. Nature 163:18
USDA “Value Added Products from Feather Fiber” $175 K (2004–2006), http://www.eng.auburn.edu/users/royalb/EXPERIENCE.htm (Accessed March 5, 2014)
USDA awards grant $500,000 to develop bio-based products http://www.udel.edu/PR/UDaily/2005/mar/rwool080405.html (Accessed March 5, 2014)
Idris A, Vijayaraghavan R, Rana UA, Fredericks D, Patti AF, MacFarlane DR (2013) Dissolution of feather keratin in ionic liquids. Green Chem 15:525–534
Fan X (2008) Value-added products from chicken feather fibers and protein. Doctoral Thesis. PhD Dissertation, Auburn University, Auburn
Tonin C, Aluigi A, Vineis C, Varesano A, Montarsolo A, Ferrero F (2007) Thermal and structural characterization of poly (ethylene-oxide)/keratin blend films. J Therm Anal Calorim 89:601–608
Aluigi A, Vineis C, Ceria A, Tonin C (2008) Composite biomaterials from fibre wastes: Characterization of wool–cellulose acetate blends. Composites Part A Appl S 39:126–132
Hameed N, Guo Q (2010) Blend films of natural wool and cellulose prepared from an ionic liquid. Cellulose 17:803–813
Iridag Y, Kazanci M (2006) Preparation and characterization of Bombyx mori silk fibroin and wool keratin. J Appl Polym Sci 100:4260–4264
Vasconcelos A, Freddi G, Cavaco-Paulo A (2008) Biodegradable materials based on silk fibroin and keratin. Biomacromolecules 9:1299–1305
Yuan J, Xing ZC, Park SW, Geng J, Kang IK, Shen J, Meng W, Shim KJ, Han IS, Kim JC (2009) Fabrication of PHBV/keratin composite nanofibrous mats for biomedical applications. Macromol Res 17:850–855
Barone JR, Schmidt WF, Liebner CF (2005) Thermally processed keratin films. J Appl Polym Sci 97:1644–1651
Barone JR, Schmidt WF, Gregoire N (2006) Extrusion of feather keratin. J Appl Polym Sci 100:1432–1442
Desnuelle P (1953) In: Neurath H, Bailey K (eds) The Proteins, vol I, Part A. Academic Press, New York, p 87
Jia QX, Xiong ZJ, Shi CM, Zhang LQ, Wang XN (2012) Preparation and properties of polyamide 6 fibers prepared by the gel spinning method. J Appl Polym Sci 124:5165–5171
Tsen C (1969) Effects of oxidizing and reducing agents on changes of flour proteins during dough mixing. Cereal Chem 46:435–442
Ghosh S, Banerjee A (2001) A Multitechnique Approach in Protein/Surfactant Interaction Study: Physicochemical Aspects of Sodium Dodecyl Sulfate in the Presence of Trypsin in Aqueous Medium. Biomacromolecules 3:9–16
Behrendt J, Arevalo E, Gulyas H, Niederste-Hollenberg J, Niemiec A, Zhou J, Otterpohl R (2002) Production of value added products from separately collected urine. Water Sci Technol 46:341–346
Wada M, Takagi H (2006) Metabolic pathways and biotechnological production of l-cysteine. Appl Microbiol Biotechnol 73:48–54
Gallezot P (2007) Process options for converting renewable feedstocks to bioproducts. Green Chem 9:295–302
Long JJ, Cui CL, Wang L, Xu HM, Yu ZJ, Bi XP (2013) Effect of treatment pressure on wool fiber in supercritical carbon dioxide fluid. J Clean Prod 43:52–58
Elsworth FF, Phillips H (1941) The action of sulphites on the cysteine disulphide linkages in wool: The influence of temperature, time and concentration on the reaction. Biochem J 35:135–143
Xu H, Cai S, Xu L, Yang Y (2014) Water-stable three-dimensional ultrafine fibrous scaffolds from keratin for cartilage tissue engineering. Langmuir 30:8461–8470
Xu H, Yang Y (2014) Controlled De-Cross-Linking and Disentanglement of Feather Keratin for Fiber Preparation via a Novel Process. ACS Sustain Chem Eng 2:1404–1410
Xu H, Cai S, Sellers A, Yang Y (2014) Electrospun ultrafine fibrous wheat glutenin scaffolds with three-dimensionally random organization and water stability for soft tissue engineering. J Biotechnol 184:179–186
Xu H, Cai S, Sellers A, Yang Y (2014) Intrinsically water-stable electrospun three-dimensional ultrafine fibrous soy protein scaffolds for soft tissue engineering using adipose derived mesenchymal stem cells. RSC Adv 4:15451–15457
Reddy N, Li Y, Yang Y (2009) Alkali-catalyzed low temperature wet crosslinking of plant proteins using carboxylic acids. Biotechnol Prog 25:139–146
Tanabe T, Okitsu N, Tachibana A, Yamauchi K (2002) Preparation and characterization of keratin–chitosan composite film. Biomaterials 23:817–825
Tanabe T, Okitsu N, Yamauchi K (2004) Fabrication and characterization of chemically crosslinked keratin films. Mater Sci Eng, C 24:441–446
Katoh K, Shibayama M, Tanabe T, Yamauchi K (2004) Preparation and physicochemical properties of compression-molded keratin films. Biomaterials 25:2265–2272
Vasconcelos A, Freddi G, Cavaco-Paulo A (2008) Biodegradable Materials Based on Silk Fibroin and Keratin. Biomacromolecules 9:1299–1305
Katoh K, Shibayama M, Tanabe T, Yamauchi K (2004) Preparation and properties of keratin–poly(vinyl alcohol) blend fiber. J Appl Polym Sci 91:756–762
Hou X, Xu H, Shi Z, Ge M, Chen L, Cao X, Yang Y (2014) Hydrothermal pretreatment for the preparation of wool powders. J Appl Polym Sci. doi:10.1002/app.40173
Tsukada M, Freddi G, Monti P, Bertoluzza A, Kasai N (1995) Structure and molecular conformation of tussah silk fibroin films: Effect of methanol. J Polym Sci, Part B: Polym Phys 33:1995–2001
Xu W, Cui W, Li W, Guo W (2004) Development and characterizations of super-fine wool powder. Powder Technol 140:136–140
Acknowledgements
This research was financially supported by the Agricultural Research Division at the University of Nebraska-Lincoln, USDA Hatch Act, Multistate Research Project S-1054 (NEB 37-037) and Nebraska Environmental Trust (13-142). The authors thank the AATCC student research grant for Zhuanzhuan Ma. The authors also thank Dr. Han Chen for his help in SEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Ma, Z. & Yang, Y. Dissolution and regeneration of wool via controlled disintegration and disentanglement of highly crosslinked keratin. J Mater Sci 49, 7513–7521 (2014). https://doi.org/10.1007/s10853-014-8457-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8457-z