Abstract
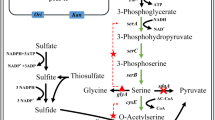
l-Cysteine is an important amino acid both biologically and commercially. Although most amino acids are commercially produced by fermentation, cysteine is mainly produced by protein hydrolysis. However, synthetic or biotechnological products have been preferred in the market. Biotechnological processes for cysteine production, both enzymatic and fermentative processes, are discussed. Enzymatic process, the asymmetric hydrolysis of dl-2-amino-Δ2-thiazoline-4-carboxylic acid to l-cysteine, has been developed and industrialized. The l-cysteine biosynthetic pathways of Escherichia coli and Corynebacterium glutamicum, which are used in many amino acid production processes, are also described. These two bacteria have basically same l-cysteine biosynthetic pathways. l-Cysteine-degrading enzymes and l-cysteine-exporting proteins both in E. coli and C. glutamicum are also described. In conclusion, for the effective fermentative production of l-cysteine directly from glucose, the combination of enhancing biosynthetic activity, weakening the degradation pathway, and exploiting the export system seems to be effective.



Similar content being viewed by others
References
Ajinomoto (2002) Company statement. Available from World Wide Web http://www.foodnavigator.com/news/news.asp?id=43352. Cited 7 Jul 2006
Awano N, Wada M, Kohdoh A, Oikawa T, Takagi H, Nakamori S (2003) Effect of cysteine desulfhydrase gene disruption on l-cysteine overproduction in Escherichia coli. Appl Microbiol Biotechnol 62:239–243
Awano N, Wada M, Mori H, Nakamori S, Takagi H (2005) Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl Environ Microbiol 71:4149–4152
Berger EA, Heppel LA (1972) A binding protein involved in the transport of cysteine and diaminopimelic acid in Escherichia coli. J Biol Chem 247:7684–7694
Cicchillo RM, Baker MA, Schnitzer EJ, Newman EB, Krebs C, Booker SJ (2004) Escherichia coli l-serine deaminase requires a [4Fe-4S] cluster in catalysis. J Biol Chem 279:32418–32425
Daßler T, Maier T, Winterhalter C, Böck A (2000) Identification of a major facilitator protein from Escherichia coli involved in efflux of metabolites of the cysteine pathway. Mol Microbiol 36:1101–1112
Denk D, Böck A (1987) l-Cysteine biosynthesis in Escherichia coli: nucleotide sequence and expression of the serine acetyltransferase (cysE) gene from the wild-type and a cysteine-excreting mutant. J Gen Microbiol 133:515–525
Franke I, Resch A, Daßler T, Maier T, Böck A (2003) YfiK from Escherichia coli promotes export of O-acetylserine and cysteine. J Bacteriol 185:1161–1166
Haitani Y, Awano N, Yamazaki M, Wada M, Nakamori S, Takagi H (2006) Functional analysis of l-serine O-acetyltransferase from Corynebacterium glutamicum. FEMS Microbiol Lett 255:156–163
Hardy PM (1985) The protein amino acids. In: Barrett GC (ed) Chemistry and biology of the amino acids. Chapman & Hall, London, pp 6–24
Harris CL (1981) Cystine and growth inhibition of Escherichia coli: threonine deaminase as the target enzyme. J Bacteriol 145:1031–1035
Hosie AHF, Poole PS (2001) Bacterial ABC transporters of amino acids. Res Microbiol 152:259–270
Hunt S (1985) Degradation of amino acids accompanying in vitro protein hydrolysis. In: Barrett GC (ed) Chemistry and biology of the amino acids. Chapman & Hall, London, pp 376–398
Ikeda M (2003) Amino acid production process. In: Scheper T, Faurie R, Thommel J (eds) Advances in biochemical engineering/biotechnology, vol. 79. Springer, Berlin Heidelberg New York, pp 1–35
Kai Y, Kashiwagi T, Ishikawa K, Ziyatdinov MK, Redkina EI, Kiriukhin MY, Gusyatiner MM, Kobayashi S, Takagi H. Suzuki E (2006) Engineering of Escherichia coli l-serine O-acetyltransferase on the basis of crystal structure: desensitization to feedback inhibition by l-cysteine. Protein Eng Des Sel 19:163–167
Kim JW, Kim HJ, Kim Y, Lee MS, Lee HS (2001) Properties of the Corynebacterium glutamicum metC gene product encoding cystathionine β-lyase. Mol Cells 11:220–225
Kobayashi S, Masui R, Yokoyama S, Kuramitsu S, Takagi H (2004) A novel metal-activated l-serine O-acetyltransferase from Thermus thermophilus HB8. J Biochem 136:629–634
Krämer R (1994) Systems and mechanism of amino acid uptake and excretion in prokaryotes. Arch Microbiol 162:1–13
Kredich NM (1996) Biosynthesis of cysteine, In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznicoff WS, Riley M, Schaechter M, Umbarger JE (eds) Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd edn. ASM, Washington DC, pp 514–527
Kredich NM, Tomkins GM (1966) The enzymatic synthesis of l-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem 241:4955–4965
Lee HS, Hwang BJ (2003) Methionine biosynthesis and its regulation in Corynebacterium glutamicum: parallel pathways of transsulfuration and direct sulfhydrylation. Appl Microbiol Biotechnol 62:459–467
Leinfelder W, Heinrich P (1997) Process for preparing O-acetylserine, l-cysteine and l-cysteine-related products. German patent number WO 97/15673
Leuchtenberger W (1996) Amino acids-technical production and use. In: Roehr M (ed) Biotechnology, vol. 6. VCH, Weinheim, pp 465–502
Nakamori S, Kobayashi S, Kobayashi C, Takagi H (1998) Overproduction of l-cysteine and l-cystine by Escherichia coli strains with a genetically altered serine acetyltransferase. Appl Environ Microbiol 64:1607–1611
Nishino K, Yamaguchi A (2001) Analysis of a complete library of putative drug transporter genes in Escherichia coli. J Bacteriol 183:5803–5812
Noji M, Inoue K, Kimura N, Gouda A, Saito K (1998) Isoform-dependent differences in feedback regulation and subcellular localization of serine acetyltransferase involved in cysteine biosynthesis from Arabidopsis thaliana. J Biol Chem 273:32739–32745
Ohmachi T, Nishino M, Kawata M, Edo N, Funaki H, Narita M, Mori K, Tamura Y, Asada Y (2002) Identification, cloning, and sequencing of the genes involved in the conversion of d,l-2-amino-Δ2-thiazoline-4-carboxylic acid to l-cysteine in Pseudomonas sp. strain ON-4a. Biosci Biotechnol Biochem 66:1097–1104
Ono B, Hazu T, Yoshida S, Kawato T, Shinoda S, Brzvwczy J. Paszewski A (1999) Cysteine biosynthesis in Saccharomyces cerevisiae: a new outlook on pathway and regulation. Yeast 15:1365–1375
Peter-Wendisch P, Stolz M, Etterich H, Kennerknecht N, Sahm H, Eggeling L (2005) Metabolic engineering of Corynebacterium glutamicum for l-serine production. Appl Environ Microbiol 71:7139–7144
Pittman MS, Corker H, Wu G, Binet MB, Moir AJG, Poole RK (2002) Cysteine is exported from the Escherichia coli cytoplasm by CydDC, an ATP-binding cassette-type transporter required for cytochrome assembly. J Biol Chem 277:49841–49849
Pizer LI (1963) The pathway and control of serine biosynthesis in Escherichia coli. J Biol Chem 238:3934–3944
Pye VE, Tingey AP, Robson RL, Moody PCE (2004) The structure and mechanism of serine acetyltransferase from Escherichia coli. J Biol Chem 279:40729–40736
Rossol I, Pühler A (1992) The Corynebacterium glutamicum aecD gene encodes a C-S lyase with α,β-elimination activity that degrades aminoethylcysteine. J Bacteriol 174:2968–2977
Ryu OH, Ju JY, Shin CS (1997) Continuous l-cysteine production using immobilized cell reactors and product extractors. Process Biochem 32:201–209
Sano K, Mitsugi K (1978) Enzymatic production of l-cysteine from dl-2-amino-Δ2-thiazoline-4-carboxylic acid by Pseudomonas thiazolinophium: optimal conditions for the enzyme formation and enzymatic reaction. Agric Biol Chem 42:2315–2321
Sano K, Eguchi C, Yasuda N, Mitsugi K (1979) Metabolic pathway for l-cysteine formation from dl-2-amino-Δ2-thiazoline-4-carboxylic acid by Pseudomonas. Agric Biol Chem 43:2373–2374
Shiba T, Takeda K, Yajima M, Tadano M (2002) Genes from Pseudomonas sp. strain BS involved in the conversion of l-2-amino-Δ2-thiazolin-4-carbonic acid to l-cysteine. Appl Environ Microbiol 68:2179–2187
Soda K (1987) Microbial sulfur amino acids: an overview. In: Jakoby WB, Griffith OW (eds) Methods in enzymology, vol.143. Academic Press, Orlando, pp 453–459
Sørensen MA, Pederson S (1991) Cysteine even in low concentrations, induces transient amino acid starvation in Escherichia coli. J Bacteriol 173:5244–5246
Sperandio B, Polard P, Ehrlich DS, Renault P, Guédon E (2005) Sulfur amino acid metabolism and its control in Lactococcus lactis IL1403. J Bacteriol 187:3762–3778
Sugimoto E, Pizer LI (1968) The mechanism of end product inhibition of serine biosynthesis. I. Purification and kinetics of phosphoglycerate dehydrogenase. J Biol Chem 243:2081–2089
Takagi H, Kobayashi C, Kobayashi S, Nakamori S (1999a) PCR random mutagenesis into Escherichia coli serine acetyltransferase: isolation of the mutant enzymes that cause overproduction of l-cysteine and l-cystine due to the desensitization to feedback inhibition. FEBS Lett 452:323–327
Takagi H, Awano N, Kobayashi S, Noji M, Saito K, Nakamori S (1999b) Overproduction of l-cysteine and l-cystine by expression of genes for feedback inhibition-insensitive serine acetyltransferase from Arabidopsis thaliana in Escherichia coli. FEMS Microbiol Lett 179:453–459
Takagi H, Yoshioka K, Awano N, Nakamori S, Ono B (2003) Role of Saccharomyces cerevisiae serine O-acetyltransferase in cysteine biosynthesis. FEMS Microbiol Lett 218:291–297
Tamura T, Nishino M, Ohmachi T, Asada Y (1998) N-Carbamoyl-l-cysteine as an intermediate in the bioconversion from d,l-2-amino-Δ2-thiazoline-4-carboxylic acid to l-cysteine by Pseudomonas sp. ON-4a. Biosci Biotechnol Biochem 62:2226–2229
Vermeji P, Kertesz MA (1999) Pathways of assimilative sulfur metabolism in Pseudomonas putida. J Bacteriol 181:5833–5837
Viljic M, Sahm H, Eggeling L (1996) A new type of transporter with a new type of cellular function: l-lysine export from Corynebacterium glutamicum. Mol Microbiol 22:815–826
Wada M, Awano N, Haisa K, Takagi H, Nakamori S (2002) Purification, characterization and identification of cysteine desulfhydrase of Corynebacterium glutamicum, and its relationship to cysteine production. FEMS Microbiol Lett 217:103–107
Wada M, Awano N, Yamazawa H, Takagi H, Nakamori S (2004) Purification and characterization of O-acetylserine sulfhydrylase of Corynebacterium glutamicum. Biosci Biotechnol Biochem 68:1581–1583
Wheeler PR, Coldham NG, Keating L, Gordon SV, Wooff EE, Parish T, Hewinson RG (2005) Functional demonstration of reverse transsulfuration in the Mycobacterium tuberculosis complex reveals that methionine is the preferred sulfur source for pathogenic Mycobacteria. J Biol Chem 280:8069–8078
Wirtz M, Hell R (2003) Production of cysteine for bacterial and plant biotechnology: application of cysteine feedback-insensitive isoforms of serine acetyltransferase. Amino Acids 24:195–203
Yamada S, Awano N, Inubushi K, Maeda E, Nakamori S, Nishino K, Yamaguchi A, Takagi H (2006) Effect of drug transporter genes on cysteine export and overproduction in Escherichia coli. Appl Environ Microbiol 72:4735–4742
Acknowledgment
This work was supported in part by a grant from Ajinomoto, Co., Inc., to H.T.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wada, M., Takagi, H. Metabolic pathways and biotechnological production of l-cysteine. Appl Microbiol Biotechnol 73, 48–54 (2006). https://doi.org/10.1007/s00253-006-0587-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0587-z