Abstract
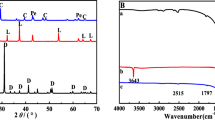
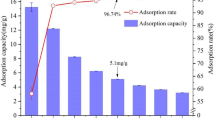
In this paper, calcite with cotton morphology was prepared by co-precipitation method using cotton as templates. The effect of contact time, initial concentration, and solution temperature on calcite adsorption of lead ions was studied using a batch technique. The maximal adsorption capacity of calcite for Pb2+ was 3347.02 mg/g with a minimum contact time of 60 min. The pseudo-second-order kinetic model and Langmuir/Freundlich isotherms were proposed for modeling kinetic and equilibrium data, respectively. Thermodynamic parameters such as ∆G 0, ∆H 0, and ∆S 0 were calculated to reveal the nature of sorption. Original calcite and the products of adsorption were characterized using SEM, TEM/EDAX, SAED, XRD, and BET. Dissolution of calcite followed by precipitation of lead carbonate was found to be the main operating mechanism for Pb2+ adsorption by calcite.








Similar content being viewed by others
References
Zhou Q, Zhao N, Xie G (2011) Determination of lead in environmental waters with dispersive liquid–liquid microextraction prior to atomic fluorescence spectrometry. J Hazard Mater 189:48–53
Mirzaei M, Behzadi M, Abadi NM, Beizaei A (2011) Simultaneous separation/preconcentration of ultra trace heavy metals in industrial wastewaters by dispersive liquid–liquid microextraction based on solidification of floating organic drop prior to determination by graphite furnace atomic absorption spectrometry. J Hazard Mater 186:1739–1743
Surme Y, Narin I, Soylak M, Yuruk H, Dogan M (2006) Cloud point extraction procedure for flame atomic absorption spectrometric determination of lead(II) in sediment and water samples. Microchim Acta 157:193–199
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
Al-Degs YS, El-Barghouthi MI, Issa AA, Khraisheh MA, Walker GM (2006) Sorption of Zn(II), Pb(II), and Co(II) using natural sorbents: equilibrium and kinetic studies. Water Res 40:2645–2658
Yavuz Ö, Guzel R, Aydin F, Tegin I, Ziyadanogullari R (2007) Removal of cadmium and lead from aqueous solution by calcite. J Environ Stud 16(3):467–471
Aziz HA, Adlan MN, Ariffin KS (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour Technol 99:1578–1583
achara JMZ, Cowan CE, Resch CT (1991) Sorption of divalent metals on calcite. Geochimica et Cosmochimica Acta 55(6):1549–1562
Cai G-B, Zhao G-X, Wang X-K, Yu S-H (2010) Synthesis of polyacrylic acid stabilized amorphous calcium carbonate nanoparticles and their application for removal of toxic heavy metal ions in water. J Phys Chem 114:12948–12954
Ma X, Li L, Yang L, Su C, Wang K, Yuan S, Zhou J (2012) Adsorption of heavy metal ions using hierarchical CaCO3-maltose meso/macroporous hybrid materials: adsorption isotherms and kinetic studies. J Hazard Mater 209–210:467–477
Ma X, Li L, Yang L, Su C, Wang K, Jiang K (2012) Preparation of hybrid CaCO3–pepsin hemisphere with ordered hierarchical structure and the application for removal of heavy metal ions. J Cryst Growth 338:272–279
KÖhler SJ, Cubillas P, Blanco JDR, Bauer C, Prieto M (2007) Removal of cadmium from wastewaters by aragonite shells and the influence of other divalent cations. Environ Sci Technol 41:112–118
Sturchio NC, Chiarello RP, Cheng L, Lyman PF, Bedzyk MJ, Qian Y, You H, Yee D, Geissbuhler P, Sorensen LB, Liang Y, Baer DR (1997) Lead adsorption at the calcite-water interface: synchrotron X-ray standing wave and X-ray reflectivity studies. Geochimicaet Cosmo Acta 61(2):251–263
Wang L, Meng Z, Yu Y, Meng Q, Chen D (2008) Synthesis of hybrid linear-dendritic block copolymers with carboxylic functional groups for the biomimetic mineralization of calcium carbonate. Polymer 49:1199–1210
Hall SR, Bolger H, Mann S (2003) Morphosynthesis of complex inorganic forms using pollen grain templates. Chem Commun 9:2784–2785
Ashtari K, Fasihi J, Mollania N, Khajeh K (2014) A biotemplated nickel nanostructure: synthesis, characterization and antibacterial activity. Mater Res Bull 50:348–353
Fan T, Sun B, Gu J, Zhang D, Lau LWM (2005) Biomorphic Al2O3 fibers synthesized using cotton as bio-templates. Scripta Mater 53:893–897
Sun B, Fan T, Xu J, Zhang D (2005) Biomorphic synthesis of SnO2 microtubules on cotton fibers. Mater Lett 9:2325–2328
Song P, Wang Q, Zhang Z, Yang Z (2010) Synthesis and gas sensing properties of biomorphic LaFeO3 hollow fibers templated from cotton. Sens Actuators B 147:248–254
Xu Q, Zheng X, Wen Z, Yang Y, Wu D, Xu M (2011) Enhanced room temperature ferromagnetism in porous BiFeO3 prepared using cotton templates. Solid State Commun 151:24–627
Xie LJ, Chu W, Huang YY, Tong DG (2011) Preparation and characterization of biomorphic nickel oxide microtubes template from cotton. Mater Lett 65:153–156
Ho YS, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76:332–340
Ho YS, Mckay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Tan IA, Ahmad AL, Hameed BH (2009) Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J Hazard Mater 164:473–482
Otero M, Rozada F, Calvo LF, García AI, Morán A (2003) Kinetic and equilibrium modelling of the methylene blue removal from solution by adsorbent materials produced from sewage sludges. Biochem Eng J 15:59–68
Li Q, Zhai J, Zhang W, Wang M, Zhou J (2007) Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J Hazard Mater 141:163–167
Coleman NJ, Brassington DS, Raza A, Mendham AP (2006) Sorption of Co2+ and Sr2+ by waste-derived 11 Å tobermorite. Waste Manag 26:260–267
Özacar M, Şengil İA (2003) Adsorption of reactive dyes on calcined alunite from aqueous solutions. J Hazard Mater 98:211–224
Prieto M, Putnisf A, Diaz LF (1993) Crystallization of solid solutions from aqueous solutions in a porous medium: zoning in (Ba, Sr)SO4. Geol Mag 130(3):289–299
Langmuir Irving (1918) The removal of cadmium and lead from aqueous solution by ion exchange with Na–Y zeolite. J Am Chem Soc 40(9):1361–1403
Özer A, Özer D (2003) Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto S. cerevisiae: determination of biosorption heats. J Hazard Mater 100:219–229
Tang W-Q, Zeng R-Y, Feng Y-L, Li X-M, Zhen W (2013) Removal of Cr(VI) from aqueous solution by nano-carbonate hydroxylapatite of different Ca/P molar ratios. Chem Eng J 223:340–346
Freundlieh HMF (1906) Uber die adsorption in Losungen. Zeitschrift fur Physikalische Chemie 57:385–470
Vasudevan S, Lakshmi J (2012) The adsorption of phosphate by graphene from aqueous solution. RSC Adv 2:5234–5242
Hasany SM, Saeed MM, Ahmed M (2002) Sorption and thermodynamic behavior of zinc(II)–thiocyanate complexes onto polyurethane foam from acidic solutions. J Radioanal Nucl Chem 252(3):477–484
Ozer A, Akkaya G, Turabik M (2006) Biosorption of acid blue 290 (AB 290) and acid blue 324(AB 324) dyes on Spirogyra rhizopus. J Hazard Mater 135:355–364
Yurtsever M, Sengil IA (2009) Biosorption of Pb(II) ions by modified quebracho tannin resin. J Hazard Mater 163:58–64
Sing KSW (1982) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 54(11):2201–2222
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57(4):603–619
Rouff AA, Elzinga EJ, Reeder RJ (2006) The effect of aging and pH on Pb(II) sorption processes at the calcite-water interface. Environ Sci Technol 40:1792–1798
Acknowledgements
This work was financially supported by the Natural Science Foundation of China (No. 21163023) and Natural Science Foundation of China (No. 21261026).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Z., Shen, Q., Zhang, Q. et al. The removal of lead ions of the aqueous solution by calcite with cotton morphology. J Mater Sci 49, 5334–5344 (2014). https://doi.org/10.1007/s10853-014-8236-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8236-x