Abstract
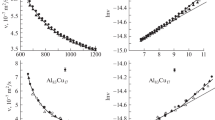
In the present work a high temperature oscillating cup viscometer has been used to measure the viscosities of liquid binary Al–Cu alloys. The dependence of viscosity on temperature is well described by the Arrhenius law. For constant temperature, the viscosity as a function of copper concentration exhibits a maximum at a mole fraction x Cu = 0.7. This might be due to a pronounced chemical short range order in the liquid phase at this composition. As the comparison of existing phenomenological models describing viscosity as a function of composition to the experimental data is unsatisfactory, a new model for the viscosity has been developed within this work based only on a few assumptions and using the enthalpy of mixing as input parameter which is easily accessible. The agreement between model calculation and experimental data is excellent.





Similar content being viewed by others
References
Brillo J, Brooks R, Egry I, Quested P (2008) High Temp High Press 37:371
Egry I (1991) J Mater Sci 26:2997. doi:10.1007/BF01124834
Schneider S, Egry I, Seyhan I (2002) Int J Thermophys 23:1241
Schmitz J, Brillo J, Egry I, Schmid-Fetzer R (2009) Int J Mater Res 100:1529
Brillo J, Plevachuk Yu, Egry I (2010) J Mater Sci 45:5150. doi:10.1007/s10853-010-4512-6
Brillo J, Egry I, Westphal J (2008) Int J Mater Res 99:162
Brillo J, Egry I (2003) Int J Thermophys 24:1155
Jones WRD, Bartlett WL (1952–1953) J Inst Met 81:145
Gebhardt E, Becker M, Dorner S (1953) Z Metallkd 44:510
Lihl F, Nachtigall E, Schwaiger A (1968) Z Metallkd 59:213
Roscoe R (1958) Proc Phys Soc 72:576
Friedrichs HA, Ronkow LW, Zhou Y (1997) Steel Res 68:209
Plevachuk Y, Sklyarchuk V, Yakymovych A, Eckert S, Willers B, Eigenfeld K (2008) Met Mater Trans A 39:3040
Konstantinova NY, Popel PS (2008) J Phys: Conf Ser 98:062022
Kehr M, Hoyer W, Egry I (2007) Int J Thermophys 28:1017
Dinsdale A, Quested PN (2004) J Mater Sci 39:7221. doi:10.1023/B:JMSC.0000048735.50256.96
Brillo J, Brooks R, Egry I, Quested PN (2007) Int J Mater Res 98:457
Moelwyn-Hughes EA (1961) Physical chemistry. Pergamon Press, Oxford
Kozlov LYa, Romanov LM, Petrov NN (1983) Izv vysch uch zav Chernaya Metallurgiya 3:7
Seetharaman S, Sichen D (1994) Met Mater Trans B 25:589
Kaptay G (2003) In: Proceedings of microCAD 2003 Conference, Section Metallurgy, University of Miscolc 23
Hirai M (1993) ISIJ Int 33:251
Ferris D, Quested PN (2002) NPL Report CMMT(A):306
Iida T, Guthrie R (1993) The physical properties of liquid metals. Clarendon Press, Oxford
Assael MJ, Kakosimos K, Banish RM, Brillo J, Egry I, Brooks R, Quested PN, Mills KC, Nagashima A, Sato Y, Wakeham WA (2006) J Phys Chem Ref Data 52:285
Brillo J, Chathoth SM, Koza MM, Meyer A (2008) Appl Phys Lett 93:121905
Witusiewicz VT, Hecht U, Fries SG, Rex S (2004) J Alloys Compd 385:133
Kehr M, Schick M, Hoyer W, Egry I (2008) High Temp High Press 37:361
Walsdorfer H, Arpshofen I, Predel B (1988) Z Metallkd 79:503
Brillo J, Bytchkov A, Egry I, Hennet L, Mathiak G, Pozdnyakova I, Price DL, Thiaudiere D, Zanghi D (2006) J Non-Cryst Solids 352:4008
Maret M, Pomme T, Pasturel A (1990) Phys Rev B 42:1598
Das SK, Horbach J, Koza MM, Mavilla Chatoth S, Meyer A (2005) Appl Phys Lett 86:011918
Budai I, Benkö MZ, Kaptay G (2005) Mater Sci Forum 473–474:309
Dinsdale AT (1991) CALPHAD 15:317
Saunders N (1998) In: Ansara I, Dinsdale AT, Rands MH (eds) COST-507 Thermochemical database for light metal alloys. European Communities, Luxemburg, p 28
Redlich O, Kister AT (1948) Ind Eng Chem 40:345
Hillert M, Staffansson LI (1970) Acta Chem Scand 24:3618
Sundman B, Ågren J (1981) J Phys Chem Solids 42:297
Acknowledgements
The support of this work by Deutsche Forschungsgemeinschaft (DFG) under Contract No. EG 93/8-1 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schick, M., Brillo, J., Egry, I. et al. Viscosity of Al–Cu liquid alloys: measurement and thermodynamic description. J Mater Sci 47, 8145–8152 (2012). https://doi.org/10.1007/s10853-012-6710-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6710-x