Abstract
Titanium dioxide nanotubes (TiO2 NTs) with various sizes have been prepared by low-temperature chemical synthesis using commercial anatase TiO2 particles with different crystallite size in NaOH solution and used as a photoelectrode in a dye-sensitized solar cell (DSSC). The relationship between the physicochemical properties of electrode materials and photovoltaic performance was investigated. The electrodes made from modified TiO2 NTs showed a strong dependency on their specific surface area and resultant amount of dye adsorption; the surface area decreased with increase in the diameter of the NT from 9.8 to 23.6 nm. The conversion efficiency of the cell made from TiO2 NT, 12.9 nm in diameter, was enhanced by 12% compared to that of the smallest NT. These results suggested that the photovoltaic performance improved by the suppression of photogenerated charge recombination in spite of a 25.3% reduction in the specific surface area. In addition, larger TiO2 NTs could be utilized as a scattering layer on the top of the TiO2 nanoparticulate working electrode. It was observed that this controlled TiO2 photoelectrode architecture exhibited enhanced conversion efficiency without TiCl4 treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, titanium oxide nanotubes (TiO2 NTs) [1–3] have been extensively studied in the field of energy creation and environmentally friendly systems. Dye-sensitized solar cell (DSSC) [4], which is a potential alternative to conventional solar cell, is considered as an application of the TiO2 NTs. For high photovoltaic performance of DSSC, there have been significant research efforts to discover the nature of this device, which usually consists of anode electrode materials, sensitized dye, electrolyte, and counter electrode. Because anode electrode materials require nanoscale and highly crystalline semiconductors with large surface area for the adsorption of sensitized dyes, various one-dimensional (1D) nanostructured TiO2 materials such as nanowire [5], nanorod [6–10], and nanotubes [11–29] have gained much attention.
In general, TiO2 NTs are synthesized for the electrodes of DSSC by the electrochemical anodic oxidation method [3, 8, 11–13] or the low-temperature chemical synthesis route [2, 14], and their various tailoring structures and morphological features were investigated and determined depending on their method of synthesis. Vertically oriented TiO2 NT arrays made by anodization of titanium in fluoride-based baths have precisely oriented nature of the NT array and excellent electron percolation pathways. However, the photovoltaic conversion efficiency [15, 16] was inferior to those of TiO2 nanoparticle-based DSSCs owing to the insufficient surface area for dye adsorption and the relatively larger number of grain boundaries among nanoparticulate TiO2 crystallites.
On the other hand, since Uchida et al. [17] reported DSSC of low-temperature chemically synthesized TiO2 NTs with the cell efficiency of 2.9%, most researches have focused on the comparative study of commercial TiO2 nanoparticles such as P25 and TiO2 NT [18–20]. Ohsaki et al. [18] reported the improved cell efficiency of 7.1% of TiO2 NT electrode by optimizing electrode preparation condition and electrolyte composition of the cell. They analyzed electron transport properties and revealed that the TiO2 NT electrode had a longer electron lifetime and resultant diffusion length than those of P25 electrode. Remarkably long lifetime of trapped electron in TiO2 NT was also revealed by the photoinduced charge separation and recombination analysis using time-resolved diffuse reflectance spectroscopy, showing the advantage of 1D nanostructured TiO2 in charge transport behavior [22]. More recently, Li et al. [20] reported the relatively high cell efficiency with TiO2 NT of 7.6%.
Regarding to the correlation between morphology of oxide photoelectrode material and its photovoltaic performance, there are a few reports on the size effect of 1D nanostructured TiO2 photoelectrode materials, which were prepared by electrochemical anodization route, on the DSSC performance [11, 12, 30]. For anodized TiO2 nanotubular photoelectrodes, Ghicov et al. [12] recently reported that smaller diameters exhibited higher efficiency and pointed the importance of its aspect ratio as well as its crystallinity. These results imply that well-controlled aspect ratio of 1D nanostructured materials can provide larger photocurrent density and also result in better efficiency. In addition, it is generally argued that the architecture of the oxide photoelectrodes such as film thickness, multi-layered structure, and so on is another important factor to be considered for enhancing the DSSC performance [7, 30]. Therefore, the optimization of the balance between the diameter and thickness of 1D nanomaterial is important and required for improving the energy conversion performance.
Recently, we synthesized TiO2 NTs with different diameters via the low-temperature solution chemical route using different sizes of raw powders [31]. However, to the best of our knowledge, the effect of the diameter of TiO2 NT synthesized by the low-temperature chemical route on the photovoltaic properties of DSSC has rarely been reported in spite of a few reports for the anodized TiO2 NTs electrode as mentioned before. The most significant difference between chemically prepared TiO2 NT and anodized one is its crystallographic feature: the former one is consisted of TiO6 octahedral network with tubular-layered compound-like structure [2, 21], while the latter one is amorphous TiO2 or aggregate of nanocrystalline TiO2 depending on the heat treatment [3], respectively. Therefore, the morphological characteristic of chemically prepared NT might strongly influence on the charge transport and/or recombination properties as the oxide photoelectrode.
In this study, we fabricated the photoelectrode using a series of different-sized TiO2 NTs synthesized by low-temperature chemical route, which have an open-end nanotubular structure, and investigated the relationships between morphological characteristics of TiO2 NTs and their photovoltaic performance as anode electrodes at two aspects. The first one is systematical study of the influence of various sized 1D nanostructured TiO2 NTs photoelectrode on the DSSC performance using the conventional single-layered electrode structure. Secondly, to investigate the major contribution of electron transport or light scattering in photovoltaic performance, we architecturally designed double-layered electrode by adapting modified TiO2 NTs as a scattering layer on the TiO2 nanoparticulate layer (here we used commercial P25 powder). Based on the results, we discuss in detail regarding the critical factor of photoelectrode in photovoltaic performance.
Experimental
Preparation of size-controlled TiO2 NTs
The TiO2 NTs with different crystallite sizes were prepared by annealing commercial anatase powders (Kojundo Chemical Lab. Co., Ltd., Tokyo, Japan) in a furnace at 500, 1000, and 1300 °C for 4 h [31]; hereafter, preannealed TiO2 powders are denoted as A, and heat-treated ones are, A 500, A 1000, and A 1300, respectively, for the above-mentioned temperatures. The TiO2 powder (1 g) and 100 mL of a 10-M NaOH aqueous solution were placed in a 250-mL Nalgene bottle and then treated in an oil bath for 62 h at 110 °C after 30 min of ultrasonication, as described in some other studies [2, 14]. To eliminate the residual Na ion in the TiO2 NTs and to prevent its influence, we performed the ion-exchange process using 0.1 M HCl and distilled water. The precipitate was filtered and dried at 70 °C for 24 h to obtain nanotubular powders (similar to A, this is also denoted as TNT, TNT 500, TNT 1000, and TNT 1300, respectively). The fundamental morphological properties are summarized in Table 1.
Fabrication of DSSC
In the present study, synthesized TiO2 NTs and commercial TiO2 P25 (Degussa, Germany) powders are used for the preparation of pastes. The pastes containing powders with ethyl cellulose and α-terpineol in ethanol were dispersed through ball milling for 24 h at 300 rpm. The films (10 μm) were deposited on the conducting F-doped tin oxide (FTO) coated glass (Pilkington TEC 8, sheet resistance 8 Ω/□) via the screen printing method. For the desired thickness of about 10 μm, the screen printing procedure was repeated four or five times and then dried for 10 min at 125 °C to evaporate volatile components of the paste. The electrodes coated with the pastes were gradually heated under an air flow at 500 °C for 30 min to remove the organic loads and to improve the interconnection of the particles. The corresponding cells are denoted by their powder names.
To investigate the role of modified TiO2 NTs as a scattering layer, the double-layered working electrode architecture was also fabricated as follows. The first layer was prepared on the FTO substrate using P25 paste prepared in a way similar to the method mentioned above. Then the modified TiO2 NTs pastes were deposited on the P25 film, maintaining the thickness of about 4 μm. The next procedure is the same as the above method. In addition, commercial paste Ti-Nanoxide R/SP (named as R hereinafter, Solaronix Inc., Switzerland), which consisted of 400-nm anatase particles, was used as a reference for the scattering layer on a pre-existing nanostructured P25 TiO2 electrode. Thus, 14-μm-thick double-layered electrodes were fabricated and named TNT/P25, TNT 500/P25, TNT 1000/P25, TNT 1300/P25, and R/P25.
The TiCl4 treatments were performed, if required, by soaking the electrodes in 40 mM TiCl4 aqueous solution at 70 °C for 30 min followed by water and ethanol rinse solutions and then heating at 450 °C for 30 min. After the electrodes naturally cooled to 80 °C, it was immersed into a 0.3-mM ethanolic solution of N719 (commercially available ruthenium-based dye [RuL2(NCS)2]:2 TBA, from Solaronix Inc., Switzerland) at room temperature for 24 h. They were then rinsed sufficiently with anhydrous ethanol to remove excess dye from the surface and air dried at room ambient.
Counter electrodes were prepared by thermal decomposition of hexacholorplatinic(IV) acid hexahydrate that was dissolved in anhydrous isopropanol. A drop of 5-mM solution was put and spread on the clean FTO substrate and dried in air. Coated electrodes were annealed at 400 °C for 30 min. The dye-sensitized electrode and the counter electrode were carefully separated by a 50-μm Surlyn sheet (Dyesol Ltd., Australia) and sealed by heating. The active area of cells was 0.25 cm2. The electrolyte solution, which composed 0.1 M LiI, 0.05 M I2, 0.6 M 1,2-dimethyl-3-propylimidazlium iodide (DMPImI), and 0.05 M tert-butylpyridine in acetonitrile, was introduced into the interspaces between cells by capillary action.
Characterization
The overall phase of raw materials and synthesized NTs were analyzed using an X-ray diffractometer (XRD, RINT 2200, Rigaku Co., Tokyo, Japan) with Cu Kα radiation (λ = 0.15418 nm). The surface morphology and microstructures of samples were investigated by field emission scanning electron microscopy (FE-SEM, S-4100L, Hitachi, Ltd., Tokyo, Japan) and high-resolution transmission electron microscopy (HR-TEM, JEM-2010, JEOL Ltd., Tokyo, Japan, 200 kV). Specific surface areas of the samples were estimated using nitrogen adsorption–desorption isotherm by the Brunauer–Emmet–Teller (BET, NOVA 1000, Quantachrome, Florida, USA) method. The samples were degassed for 1 h at 105 °C under vacuum. Optical spectra at ultraviolet (UV) and visible (vis) regions of the electrode films were recorded (V-670 UV–VIS–NIR Spectrophotometer, JASCO, Japan) to investigate the optical properties.
Sheet resistivity measurements for the prepared photoelectrodes were performed by the van der Pauw method (Resitest 8300, Toyo Corporation, Tokyo, Japan) at temperatures from room to 600 °C to evaluate the electrical properties of the electrodes and to determine the annealing temperature of the oxide photoelectrode layer; oxide layers were screen-printed onto the pure glass substrate to eliminate the conduction in the FTO surface layer and then used to measure the resistivity up to the temperature. The photocurrent–voltage characteristics of the cells were measured using a digital source meter (Keithley model 2400, Tokyo, Japan) by applying external potential bias to the cell under illumination through light with an energy of 100 mW/cm2 (OTENTO-SUNIII, Bunkoh-Keiki, Tokyo, Japan). The light intensity of the illumination source was calibrated using a standard silicon photodiode (BS520, Bunkoh-Keiki, Tokyo, Japan).
Results and discussion
Morphological characteristics of materials for anode electrode
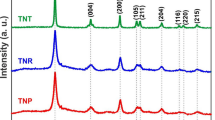
The electrode materials for DSSCs were synthesized by low-temperature chemical synthesis using commercial anatase TiO2 nanoparticles, which were obtained at various annealing temperatures. Figure 1 shows their enlarged XRD peaks at (101) and (110). As the annealing temperature increased, grain growth occurred in the powder. XRD analysis revealed that when the annealing temperature was below 650 °C, the samples contained the anatase phase. Both anatase and rutile phases appeared in the present sample annealed at 1000 °C implying that phase transformations occurred in the temperature range 600–1100 °C, depending on the preparation conditions, particle size, and the presence of impurities [32–34]. On the other hand, the anatase phase disappeared at an annealing temperature of 1300 °C, indicating that the complete anatase to rutile phase transformation occurred between these temperatures (1000–1300 °C). The average crystallite size was estimated from the line broadening of the X-ray diffraction reflections of the (101) and (110) peaks of anatase and rutile TiO2, respectively, using the Sherrer formula [35]. As can be seen from Fig. 1 and Table 1, increasing the annealing temperature increased the crystallinity of TiO2 powder and, accordingly, the growth of TiO2 crystallites.
Figure 2 shows TEM images of as-synthesized TiO2 NTs, indicating crystallite size dependency of the starting materials on the product morphology. The diameter of individual NTs is 9.8 nm (a), 12.9 nm (b), 19.1 nm (c), and 23.6 nm (d), respectively. Even though TNT 1300 shows the form of overlapped NTs owing to the aggregation tendency of TiO2 NTs (see ESM, Fig. 1S), the diameter of a single NT is more than two times the diameter of TNT. Therefore, the typical diameter of synthesized TiO2 NTs is proportional to the TiO2 particle size used for chemical synthesis.
Figure 3 shows the adsorption–desorption isotherms of as-prepared TiO2 NTs samples for synthesis duration of 62 h. The isotherms indicate a typical IUPAC type IV patterns, suggesting that pore volume decreases as the crystallite size of raw materials increase. The hysteresis loops of TNT and TNT 1000 are similar to those of TNT 500 and TNT 1300, respectively. From the BET curves, it could be inferred that the larger diameter of TiO2 NTs might not be suitable for photoelectrode materials, because specific surface area is directly related to the amount of dye adsorption, and it would result in the decrease of short current density (J sc) and conversion efficiency of cell.
Table 1 summarizes morphological property comparisons of modified TiO2 NTs. Crystallite sizes of raw materials that were estimated through XRD measurement [31] are closely related to the typical diameter of individual TiO2 NTs (measured from TEM images) and specific surface area of synthesized TiO2 NTs. Consequently, it is reasonably considered that the morphological characteristics can be controlled by the synthesis condition of the present oxide NT.
Electrical properties of oxide photoelectrodes
Figure 4 shows the temperature dependency of sheet resistivity of anode photoelectrodes (i.e., P25 and representative modified TiO2 NT, TNT 500) with a thickness of 10 μm, which was prepared on the insulating glass substrate to eliminate the effect of FTO coating. The resistivity was measured at temperatures from room to 600 °C during the heating and cooling process. It is essentially important to control the resistivity of the bulk electrode, which is an important parameter that decreases cell efficiency. In particular, as the shape of the material or morphological properties possibly affect the results, it is worthwhile to investigate the sheet resistivity of the electrode consisting of nanocrystalline oxide materials with high intrinsic resistivity. Four-point-measurement techniques, such as the van der Pauw method [36], are suitable to investigate the effect caused by the bulk as well as the overall resistivity of the electrode samples.
It was revealed that sheet resistivity of both P25 and TNT 500 electrodes decreased with an increase in the test temperatures; this behavior was also confirmed for the other modified TiO2 NTs (not shown in Fig. 4). This variation is considered because of the intrinsic nature of the semiconductor material as well as the elimination of organic components that were used to prepare the coating paste and that followed improvement of the mechanical contact of particles (thus sintering of oxides). The sheet resistivity values of P25 were mostly lower than those of TNT 500 regardless of heating, and for both cases, the resistivity after heating was lower because of the consolidation of the oxide layers. However, after returning to room temperature, the sheet resistivity of the TNT 500 electrode exhibited more than two orders of lower value than before, while that of P25 was around two orders of decrease in magnitude.
These characteristics of sheet resistivity for P25 and modified TiO2 NT may result from the essential difference between three-dimensional (3D) spherical nanoparticles and 1D NTs; nanoparticulate material generally has better sinterability owing to the equiaxial shape, smaller size, and resultant high driving force of coalescence. In other words, higher heating temperature results in a dense nanoparticulate oxide layer. In the case of 1D nanomaterials, however, sinterability is considered to be lower than the nanoparticulate system, implying that the NTs can maintain higher porosity and surface area. All these structural features affect the total conversion efficiency in photovoltaic electrode applications. In the present TiO2 systems, although the sheet resistivity of electrodes decreased depending on the heating temperature, it should, however, be noted that the surface area also significantly decreased after sintered at 500 °C, which would result in the decreased amount of the adsorbed dye. Therefore, it is important to optimize the annealing temperature at which better crystallinity and high surface area achieved, while excess neck growth between the NTs should be prevented. The annealing temperature was then fixed to be 500 °C, which was determined after SEM and BET analyses for the samples annealed at different temperatures by considering the above-mentioned morphological features and the specific surface area of samples.
J–V curves of modified TiO2 NT DSSC
Figure 5 shows the photocurrent density–voltage (J–V) curves of single-layered electrodes with modified TiO2 NTs after the TiCl4 treatment. The J–V curves under the dark condition are also plotted. Their DSSC performances which were obtained from each J–V curve as well as characteristic values are summarized in Table 2.
The overall cell efficiency (η) and fill factor (FF) for the DSSCs coated with various samples are calculated as the following equation.
where the V oc is the open circuit voltage, J sc is the short circuit current density, P input is the intensity of the incident light (In this experiment, the P input is fixed at 100 mW/cm2), and FF is defined as the equation
where the V max × J max is the maximum power point, P max the product of J–V at the maximum power point. Among the investigated samples, TNT 500 exhibited the highest cell performance, presenting a J sc, V oc, FF, and cell efficiency of 12.8 mA/cm2, 0.683 V, 0.668 and 5.83%, respectively. It is reasonably understood that TNT 500 shows the highest cell efficiency because it has sufficient surface area and amount of adsorbed dye. Furthermore, it is likely that conversion efficiency is directly proportional to the amount of adsorbed dye, specific surface area, and J sc. However, considering the fact that TNT 500 demonstrated smaller specific surface area and amount of adsorbed dye than those of TNT, there might be another factor that more dominantly affects the cell performance, specifically on the electron transport properties.
Although it is not a direct method to detect the recombination process, the comparison of the dark currents of samples could provide information about the formation of the energy barrier on the surface of the electrode [37, 38]. However, in the present DSSCs, at high forward bias (>0.4 V), dark current values decreased with an increase in the diameter of the modified TiO2 NTs; at the same bias voltage, the dark current value decreased with an I3 − ions on the electrode surface for the larger diameter of TiO2 NTs. In other words, the larger size of TiO2 NT results in the degradation of the surface area for adsorption of iodide and, consequently, promotes a decrease in the number of the surface recombination sites and resultant V oc increase [39].
In addition, the decrease in the dark current with greater diameter is attributed to a rise in V oc. This is in accordance with Eq. 3, which shows that V oc is mainly related to the electron density in the conduction band [40] and the number of surface recombination sites:
where k and T are the Boltzmann constant and the cell temperature, respectively. e is the charge on the electron, \( J_{\text{inj}} \) is the injected electron current, n cb is the concentration of electrons at the TiO2 surface, and k et is the rate constant for the reduction of I3 − by conduction band electrons. From the above equation, it can be assumed that V oc is inversely proportional to the recombination rate, and the greater diameter of TiO2 NT results in an increase of V oc. The injection rate of electrons to the larger diameter of TiO2 NT surfaces was also reduced owing to the decreased amount of the adsorbed dye. Therefore, despite of drop in J sc, V oc increased, and there was compensation for concentration of electrons at the TiO2 surface or reduction in I3 − by electrons in the conduction band of the oxide NT photoelectrodes.
J–V curves of double-layered DSSC
In general, double-layered electrode architecture, which consists of transparent layer (TiO2 particle size is about 20 nm) and light scattering layer (400 nm), is considered to improve the cell performance by increasing the amount of adsorbed dye on the surface of electrode and the light scattering effect in the electrode [41]. Figure 6 shows the J–V curves of double-layered electrodes fabricated with modified TiO2 NTs on the P25 electrode (a) before and (b) after TiCl4 treatment. In the case of modified TiO2 NT with a single-layered electrode, the contribution of a larger diameter of TiO2 NT on the photovoltaic performance is not very significant; however, it is worthwhile to investigate the photovoltaic characteristics of modified TiO2 NTs as a light scattering layer.
Because the diameter of TNT 1300 was more than two times larger than that of TNT and its specific surface area was reduced as discussed in BET results (see Table 1 and Fig. 3), dye absorbable sites would certainly be decreased. However, when the modified TiO2 NTs are deposited as a double-layered structure on the P25 nanoparticle electrode, it is expected that the cell efficiency will be affected by the excited electron transport or trapping of incident light.
The J sc, V oc, FF, and conversion cell efficiency of the double-layered DSSC are summarized in Table 3. Photovoltaic results before TiCl4 treatment shown in Fig. 6a exhibited that TNT 1300/P25 had the best performance among the modified TiO2 NT photoelectrodes, and the cell efficiency exhibited the proportional dependency on the diameter of TiO2 NT. When the P25 underlayer was incorporated to the TNT 1300 layer (TNT 1300/P25), the J sc increased from 8.82 to 11.6 mA/cm2, and the conversion cell efficiency was enhanced from 3.97 to 5.12% in comparison to those of single-layered geometry (TNT 1300, see Table 2) that corresponded to each increment of 31.3 and 29.0%, respectively. This is mainly ascribed to the light scattering effect in adequately enlarged TiO2 NTs incorporated in the electrode, which may lead to enhancement of the photoresponse to infrared light [42]. In single-layered modified TiO2 NT, the cell efficiency was decreased along with increasing diameter (Table 2) because of the reduction of adsorbed dye. Regardless of similar materials, however, the TiO2 NTs play a significant role in improving the cell efficiency (Table 3) when they are combined with the 10-μm-thick P25 underlayer electrode, probably owing to the trapping of the incident light. With respect to the morphological characteristics of modified TiO2 NTs, it is possible to infer that electron transport might not be improved because it is not well-connected to the interface between P25 and TiO2 NT owing to packing difficulty. In addition, the closed-packing, which could be explained in terms of packing density of the photoelectrodes, is more difficult for NTs than that for nanoparticles. This was indirectly verified by the sheet resistivity of the electrode, showing the intrinsic structural difference between 3D spherical nanoparticles and 1D NTs as previously discussed.
The main role of large particles incorporation onto the transparent oxide electrode is certainly to enhance the photoresponse of cell to infrared (IR) light, because Ru dyes (N719) have low light absorption capability in the IR region [42]. The widely used scattering layer material is anatase TiO2 consisting of 400-nm-sized particles on top of the active layer as stated previously. The scattering layer material should be well considered in the fact of both improvement of light harvesting efficiency and counteract such as reduction of surface area and light loss due to back-scattering. The change in the refractive index between the active layer and the scattering layer as well as the chemical bonding strength between particles and N719 are also critical factors.
As mentioned before, physical and optical properties as well as architectures of photoelectrode oxides play critical roles in improving the cell efficiency by contributing to electron transport path and light scattering effect. The double-layered structure of TiO2 NTs on TiO2 nanoparticles (P25) in the present study definitely showed the diameter effect of TiO2 NTs. From UV–Vis reflectance spectra analysis for the TiO2 NTs with various sizes and nanoparticles (P25), it is found that the reflectance of TiO2 NTs becomes higher as the diameter of NT increases (see ESM, Fig. 2S). It is rationalized that higher reflectance contributes to the enhanced light scattering and resultant increase in light absorption by dye molecules, which phenomena finally enhance the photovoltaic efficiency. However, with respect to the structural feature, 3D material 400-nm-sized anatase “R” seems superior to 1D TiO2 NTs, since the photovoltaic properties of R/P25 showed slightly higher performance than that of 1D TiO2 NTs (Table 3). More specifically, J sc and amount of adsorbed dye are lower than those of the other TiO2 NTs electrodes (Table 3a). These factors mean that the scattering effect is dominant in R/P25 system. This might be ascribed by the size and morphology of primary particle. Even though TiO2 NTs existed as aggregates with high aspect ratio in the electrode film, the largest diameter of TiO2 NT, TNT 1300, is only 23.6 nm in TEM observation, which is comparatively smaller than 400 nm TiO2 particle (R) as well as smaller than the wavelength of visible light. Nevertheless, the fact that TNT/P25 system has almost similar efficiency to R/P25 cell implies that TiO2 NTs used as scattering layer have sufficient amount of adsorbed dye on the surface owing to their high specific surface area.
Furthermore, by comparing the overall V oc of single- and double-layered electrodes, it was observed that the double-layered structure decreased owing to an increase in the electrode thickness. The behavior of V oc in the double-layered structure also showed the opposite tendency to the single-layered electrode; both V oc and conversion cell efficiency tended to increase with the larger diameter of TiO2 NT, which was dissimilar to the single-layered electrode.
On the other hand, it was revealed that the conversion cell efficiency became consistent in the range from 5.55 to 5.83% after TiCl4 treatment. This is regarded as the contribution of the TiCl4 treatment, which is thought to deposit a layer of TiO2 1–2-nm thick on the surface of the porous film [43]. In other words, when the diameter of the modified TiO2 NT in the second layer on the P25 underlayer was larger, the role of TiO2 NT electrode as a scattering layer could be more effective than that of the connection of particle grains; whereas, when the diameter was smaller, the role of TiO2 NTs as a scattering layer was less effective. However, the electron transport was improved by TiCl4 treatment because of the improvement in connections between the particle grains present in a thick nanoporous layer. Thus, charge recombination may be more suppressed, when the diameter of TiO2 NTs can be selected appropriately for the improvement of the electron pathway and the reduction of light loss.
Conclusion
The modified TiO2 NTs, which have successfully been synthesized using TiO2 anatase powder with different crystallite sizes, were utilized as photoanodes for DSSC. When TNT 500 having 12.9 nm diameter was used as a single-layered electrode, the DSSC demonstrated the highest cell efficiency and photocurrent density. This is ascribed to the enhancement of electron transport with increased TiO2 NT diameter without serious degradation of specific surface area.
On the other hand, the largest diameter of TiO2 NT (TNT 1300, d = 23.6 nm) exhibited lower photovoltaic performance because accelerating the electron transport was not sufficient owing to the reduced specific surface area. These results indicate that it is important to understand not only the influence of 1D morphological property on the photovoltaic performance but also the well-defined size-shaped aspect ratio of electrode materials. However, it was found that larger diameter, such as TNT 1300, was suitable for the double-layered electrode architecture without TiCl4 treatment. This observed improvement in the photovoltaic performance is attributed to the contribution of light scattering effects, which improves the light absorption rather than the electron transport.
References
Hoyer P (1996) Langmuir 12(6):1411
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1998) Langmuir 14(12):3160
Gong D, Grimes CA, Varghese OK, Hu WC, Singh RS, Chen Z, Dickey EC (2001) J Mater Res 16(12):3331
Oregan B, Gratzel M (1991) Nature 353(6346):737
Adachi M, Murata Y, Takao J, Jiu JT, Sakamoto M, Wang FM (2004) J Am Chem Soc 126(45):14943
Chou TP, Fryxell GE, Li XS, Cao G (2004) Proc SPIE 5510:129
Jiu JT, Isoda S, Wang FM, Adachi M (2006) J Phys Chem B 110(5):2087
Liu B, Aydil ES (2009) J Am Chem Soc 131(11):3985
Kim EY, Choi H, Whang CM (2010) J Mater Sci 45(14):3895. doi:10.1007/s10853-010-4448-x
Attar AS, Ghamsari MS, Hajiesmaeilbaigi F, Mirdamadi Sh, Katagiri K, Koumoto K (2008) J Mater Sci 43(17):5924. doi:10.1007/s10853-008-2872-y
Mor GK, Varghese OK, Paulose M, Shankar K, Grimes CA (2006) Sol Energy Mater Sol Cells 90(14):2011
Ghicov A, Albu SP, Hahn R, Kim D, Stergiopoulos T, Kunze J, Schiller CA, Falaras P, Schmuki P (2009) Chem-Asian J 4(4):520
Asmatulu R, Karthikeyan A, Bell DC, Ramanathan S, Aziz MJ (2009) J Mater Sci 44:4613–4616. doi:10.1007/s10853-009-3703-5
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1999) Adv Mater 11(15):1307
Shankar K, Mor GK, Prakasam HE, Yoriya S, Paulose M, Varghese OK, Grimes CA (2007) Nanotechnology 18(6):065707 (11 pp)
Zhu K, Neale NR, Miedaner A, Frank AJ (2007) Nano Lett 7(1):69
Uchida S, Chiba R, Tomiha M, Masaki N, Shirai M (2002) Electrochemistry 70(6):418
Ohsaki Y, Masaki N, Kitamura T, Wada Y, Okamoto T, Sekino T, Niihara K, Yanagida S (2005) Phys Chem Chem Phys 7(24):4157
Wei MD, Konishi Y, Zhou HS, Sugihara H, Arakawa H (2006) J Electrochem Soc 153(6):A1232
Li XD, Zhang DW, Sun Z, Chen YW, Huang SM (2009) Microelectron J 40(1):108
Chen Q, Zhou WD, Du DH, Peng LM (2002) Adv Mater 14(17):1208
Tachikawa T, Tojo S, Fujitsuka M, Sekino T, Majima T (2006) J Phys Chem B 110(29):14055
Ruan CM, Paulose M, Varghese OK, Mor GK, Grimes CA (2005) J Phys Chem B 109(33):15754
Stergiopoulos T, Ghicov A, Likodimos V, Tsoukleris DS, Kunze J, Schmuki P, Falaras P (2008) Nanotechnology 19(23): 235602 (7 pp)
Alivov Y, Fan ZY (2010) J Mater Sci 45(11):2902. doi:10.1007/s10853-010-4281-2
Lei B-X, Liao J-Y, Zhang R, Wang J, Su C-Y, Kuang D-B (2010) J Phys Chem C 114(35):15228
Zhu K, Neale NR, Halverson AF, Kim JY, Frank AJ (2010) J Phys Chem C 114(32):13433
Lopez-Ayala S, Rincon ME, Pfeiffer H (2009) J Mater Sci 44(15):4162. doi:10.1007/s10853-009-3617-2
Elsanousi A, Zhang J, Fadlalla HMH, Zhang F, Wang H, Ding X, Huang Z, Tang C (2008) J Mater Sci 43(11):7219. doi:10.1007/s10853-008-2947-9
Ong KG, Varghese OK, Mor GK, Shankar K, Grimes CA (2007) Sol Energy Mater Sol Cells 91(4):250
Kim J-Y, Sekino T, Park DJ, Tanaka S-I (2010) J Nanoprat Res. doi:10.1007/s11051-010-9990-6
Ovenstone J, Yanagisawa K (1999) Chem Mater 11(10):2770
Djaoued Y, Bruning R, Bersani D, Lottici PP, Badilescu S (2004) Mater Lett 58(21):2618
Kolen’ko YV, Garshev AV, Churagulov BR, Boujday S, Portes P, Colbeau-Justin C (2005) J Photochem Photobiol A Chem 172(1):19
Cullity B (1978) Elements of X-ray diffraction. Addison-Wesley, Reading
Van der Pauw LJ (1958) Philips Research Reports 13:1
Chen SG, Chappel S, Diamant Y, Zaban A (2001) Chem Mater 13(12):4629
Liu ZY, Pan K, Liu M, Wang MJ, Lu Q, Li JH, Bai YB, Li TJ (2005) Electrochim Acta 50(13):2583
Schlichthorl G, Huang SY, Sprague J, Frank AJ (1997) J Phys Chem B 101(41):8141
Zaban A, Greenshtein M, Bisquert J (2003) ChemPhysChem 4(8):859
Ito S, Murakami TN, Comte P, Liska P, Gratzel C, Nazeeruddin MK, Gratzel M (2008) Thin Solid Films 516(14):4613
Wang ZS, Kawauchi H, Kashima T, Arakawa H (2004) Coord Chem Rev 248(13–14):1381
O’Regan BC, Durrant JR, Sommeling PM, Bakker NJ (2007) J Phys Chem C 111(37):14001
Acknowledgements
This work was supported by the Global COE (Center of Excellence) Program, “Materials Integration (International Center of Education and Research), Tohoku University,” MEXT (Ministry of Education, Culture, Sports, Science and Technology), Japan, and partly supported by the Japan Society for the Promotion of Science (JSPS) under the Grant-in-Aid for Scientific Research (A). The authors gratefully acknowledge Prof. T. Sato and Prof. S. Yin (IMRAM, Tohoku Univ.) for their support of surface area measurement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kim, JY., Sekino, T. & Tanaka, SI. Influence of the size-controlled TiO2 nanotubes fabricated by low-temperature chemical synthesis on the dye-sensitized solar cell properties. J Mater Sci 46, 1749–1757 (2011). https://doi.org/10.1007/s10853-010-4994-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4994-2