Abstract
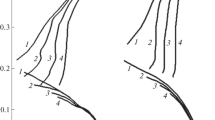
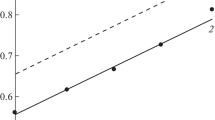
The corrosion behaviour of nitrogen-containing austenitic stainless steel in methanol containing different concentrations of H2SO4, HCl, LiCl and H2SO4 + HCl has been investigated using a potentiostatic polarization method. The cathodic reaction in the H2SO4, HCl and H2SO4 + HCl solutions was proton reduction whereas in the neutral LiCl solution, oxygen reduction was the predominant cathodic reaction. Active, passive and transpassive behaviours were observed only for higher concentrations of H2SO4 (0.01–2.0 M) due to the inherent water content. A cathodic loop, characterized by measured negative current in the anodic region, was also observed in solutions, in which the concentration of H2SO4 was 1.0 M or higher. The relative stability of the passive films decreased as the H2SO4 concentration increased, and thus the steel suffered from mild pitting corrosion. In the chloride environment, the rate of corrosion increased as the Cl− ion concentration increased. The presence of acid along with Cl− ions enhanced corrosion, and the corrosion rate increased significantly. The steel suffered from mild intergranular corrosion in acidic chloride solutions of methanol. In the H2SO4 + HCl solutions, passive films were only formed when the H2SO4 to HCl concentration ratio was greater than ∼10:1.




Similar content being viewed by others
References
Vanini AS, Audouard JP, Marcus P (1994) Corros Sci 36:1825
Flis J, Kuczynska M (2004) J Electrochem Soc 151:B573
Leckie HP, Uhlig HH (1966) J Electrochem Soc 113:1262
Hermas AA, Ogura K, Takagi S, Adachi T (1995) Corrosion 51:3
Bandy R, Van Rooyen D (1983) Corrosion 39:227
Bayoumi FM, Ghanem WA (2005) Mater Lett 59:3311
Mudali UK, Reynders B, Stratmann M (1999) Corros Sci 41:179
Tsai WT, Reynders B, Stratmann M, Grabke HJ (1993) Corros Sci 34:1647
Clayton CR, Halada P, Kearns R (1995) Mater Sci Eng A 198:135
Pawlick LA, Kelly RG (1995) J Corros Sci Eng 1:15
Singh VB, Upadhyay BN (1998) Corros Sci 40:705
Singh VK, Singh VB (1988) Corros Sci 28:385
Singh VB, Gupta A (2005) Ind J Chem Tech 12:347
Keller P, Strehblow HH (2004) Corros Sci 46:1939
Wilde BE, Hodge FG (1969) Electrochim Acta 14:619
Rozenfeld IL (1981) Corrosion 37:371
Greene ND (1960) J Electrochem Soc 107:4571
Lee JW, Osseo-Asare K, Pickering HW (1985) J Electrochem Soc 132:550
Frankenthal RP (1967) J Electrochem Soc 114:542
Hermas AA, Ogura K, Adachi T (1995) Electrochim Acta 40:837
Mazza F, Torchio S, Ghislandi N (1984) In: Proceedings of the 9th international congress on metallic corrosion, Toronto, vol. I, p 102
Szklarska -Smialowska Z, Mankowski J (1982) Corros Sci 22:1105
Umebayashi R, Akao N, Hara N, Sugimoto K (2003) J Electrochem Soc 150:B295
Zhang YS, Zhu XM (1999) Corros Sci 41:1817
Yang WP, Costa D, Marcus P (1994) J Electrochem Soc 141:2669
Asami K, Hashimoto K, Musumoto T, Shimodaira S (1976) Corros Sci 16:909
Clayton CR, Olefjord I (2002) In: Corrosion mechanism in theory and practice. Marcel Dekker, New York, p 217
Mansfeld F (1973) J Electrochem Soc 120:188
El-Naggar MM (2006) Applied Surf Sci R52:6179
Szklarska-Smialowska Z (1984) In: Proceedings of the 9th international congress on metallic corrosion, Toronto, vol. II, p 112
Tromans D, Ahmed T (1998) J Electrochem Soc 145:601
Cerquetti A, Mazza F (1973) Corros Sci 13:337
Badawy WA, Ismail KM, Fathi M (2005) Electrochim Acta 50:3603
Pistorius PC, Burstein GT (1992) Corros Sci 33:1885
Van Muylder J, Dezoubov N, Pourbaix M (1962) Report 101, CEBELCOR, Brussels, Belgium
Ujiro T, Satoh S, Staehle RW, Smyrl WH (2001) Corros Sci 43:2185
Ogura S, Sugimoto K, Sawada Y (1976) Corros Sci 16:323
Singh DDN, Gaur B, Ghosh R, Singh BK (1998) NML Tech J 40:77
Carranza RM, Alvarez MG (1996) Corros Sci 38:909
Barbucci A, Cerisola G, Cabot PL (2002) J Electrochem Soc 149:B534
Acknowledgement
Financial assistance for this work from Council of Scientific and Industrial Research (CSIR), New Delhi, India is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, V.B., Ray, M. The electrochemical behaviour of nitrogen-containing austenitic stainless steel in methanolic solution. J Mater Sci 42, 8279–8286 (2007). https://doi.org/10.1007/s10853-007-1644-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1644-4