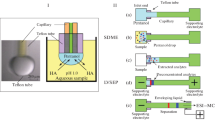
Abstract
Capillary electrophoresis is an establishing separation technique for a wide spectrum of analytes, ranging from small inorganic ions to macromolecules and it provides reliable data, requires minimal sample preparation and offers a high degree of automation. Application of crown ethers in the capillary electrophoresis because of their unique characteristics is well established. In this review article we investigated on the application of crown ethers in organic and inorganic compounds separations.








Similar content being viewed by others
References
Kuhn, R.,:Enantiomeric separation by capillary electrophoresis using a crown ether as chiral selector. Electrophoresis, 20, 2605-2613 (1999)
Rounaghi, G.H.: Mohammad ZadehKakhki, R.: thermodynamicstudy of complex formation between dibenzo-18-crown-6 and UO2cation in different non-aqueous binary solutions. J. Incl. Phenom. Macrocycl. Chem. 63, 117–122 (2009)
Rounaghi, G.H., Zavar, M.H., Mohammad ZadeKakhki, R.:Thermodynamicbehaviour of complexation process betweenDB18C6 with K + , Ag + , NH4 + and Hg2 + cations in ethylacetate-dimethylformamide binary media. Russ J Coord Chem 34, 167–171 (2008)
Rounaghi, G.H., Mohajeri, M., Soruri, F., MohamadzadehKakhki, R.: Solvent influence upon complex formation between dibenzo-18-crown-6 with the Y3+ metal cation in pure and binary mixed organic solvents. J. Chem. Eng. Data 56, 2836–2840 (2011)
Razghandi, F., Rounaghi, Gh, MohammadzadehKakhki, R.: Complexation study of dibenzo-18-crown-6 with UO2 2+Cation in binary mixed non-aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 73, 279–286 (2012)
Rounaghi, G.H., Mohajeri, M., Atashi, Z., Mohamadzadehkakhki, R.: Conductometric study of complexation reactionbetween 15-crown-5 and Cr3+, Mn2+ and Zn2+ metal cations in pure and binary mixed organic solvents. J. Incl. Phenom. Macrocycl. Chem. 73, 435–441 (2012)
Rounaghi, G.H., MohammadzadehKakhki, R.: Highly Selective and Sensitive Coated-Wire Yttrium (III) Cation Selective Electrode Based on Kryptofix-22DD. J. Electrochemical Society 158, F121–F125 (2011)
Ronaghi, G.H., Mohammadzadehkakhki, R., Sadeghian, H.: A new cerium (III) ion selective electrode based on 2,9-dihydroxy-1,10-diphenoxy-4,7-dithia decane, a novel synthetic ligand. Electrochim. Acta 101, 9756–9761 (2011)
Mohammadzadeh kakhki, R. and Rounaghi, Gh.,:Selectiveuranylcation detection by polymeric ion selective electrode based on benzo-15-crown-5,Materials Science and Engineering C,31, 1637-1642 (2011)
Mohammadzadeh Kakhki, R., Rounaghi, G.H.: Competitive bulk liquid membrane transport of heavy metal cations using the 18-crown-6 ligand as an ionophore. J. Chem. Eng. Data 56, 3169–3174 (2011)
Rounaghi, Gh, Mohammadzadeh Kakhki, R., Eshghi,H, Efficient transport of lead(II) cations in natural water using a liquid membrane system with dicyclohexano-18-crown-6 as carrier, Arabian Journal of Chemistry (2012) in press
Paikb,M.J., Kangc,J.S., Huangd,B.S., Careyd,J.R., Lee,W., Development and application of chiral crown ethers as selectors for chiral separation in high-performance liquid chromatography and nuclear magnetic resonance spectroscopy, J. Chromatogr. A, 1274 1– 5(2013)
Mohammadzadeh Kakhki, R.: Application of crown ethers as stationary phase in the chromatographic methods. J. Incl. Phenom. Macrocycl. Chem. 75, 11–22 (2013)
Mohammadzadeh Kakhki, R, Recent developments in microextraction techniques based on crown ethers. J Incl. Phenom. Macrocycl. Chem,76, 253–261(2013)
Rouhi, M.: Chiral Chemistry. Chem. Eng. News 82, 47–62 (2004)
Armstrong, D.W., Chang, L.W., Chang, S.S.C.: Mechanism of capillary electrophoresis enantioseparations using a combination of an achiral crown ether plus cyclodextrins. J. Chromatogr. A 793, 115–134 (1998)
Altria, K.D., Kelly, M.A., Clark, B.J.:Current applications in the analysis of pharmaceuticals by capillary electrophoresis trends in analytical chemistry, 17,214–226(1998)
Hohne, E., Krauss, G.J., Gubitz, G.: Capillary zone electrophoresis of the enantiomers of aminoalcohols based on host-guest complexation with a chiral crown ether J. High Resol. Chromatogr. 15, 698–700 (1992)
Kuhn, R., Steinmetz, C., Breuter, T., Hass, P., Ern, F.: Enantiomerc separations in capillary zone electrophoresis using a chiral crown ether. J. Chromatogr. A 666, 367–373 (1994)
Tanaka, Y., Otsuka, K., Terabe, S.H.: Separation of enantiomers by capillary electrophoresis–mass spectrometry employing a partial filling technique with a chiral crown ether. J. Chromatogr. A 875, 323–330 (2000)
Huang, W.X., Xu, H., Fazio, S.D., Vivilecchia, R.V.: Achievement of enantioselectivity of non-polar primary amines by a non-chiral crown ether. J. Chromatogr. A 781, 129–137 (1997)
Huang, W.X., Xu, H., Fazio, S.D., Vivilecchia, R.V.: Chiral separation of primary amino compounds using a non-chiral crown ether with beta-cyclodextrin by capillary electrophoresis. J. Chromatogr. B 695, 157–167 (1997)
Park, K.K., Kim, Y.S., Jung, H.K., Song, H.E. Park, J.W.,:Synthesis of Diaza-18-Crown-6-Functionalized b-Cyclodextrin Derivativesat the Secondary Side and Induced Circular Dichroism Studies of Their Complexes with (2-Naphthoxy)alkylammonium Ions, Bull.Kor.Chem. Soc. 21 1119-1124 (2000)
Huang, W.X., Xu, H., Fazio, S.D., Vivilecchia, R.V.: Enhancement of chiral recognition by formation of a sandwiched complex in capillary electrophoresis. J. Chromatogr. A 875, 361–369 (2000)
Koide, T., Ueno, K.: Mechanistic study of enantiomeric recognition of primary amino compounds using an achiral crown ether with cyclodextrin by capillary electrophoresis and nuclear magnetic resonance. J. Chromatogr. A 923, 229–239 (2001)
Timm, M., Jørgensen Bo, M.: Simultaneous determination of ammonia, dimethylamine, trimethylamine and trimethylamine- n -oxide in fish extracts by capillary electrophoresis with indirect UV-detection. Food Chem. 76, 509–518 (2002)
Blanco,M.; Valverde, I.,: Choice of chiral selector for enantioseparation by capillary electrophoresis, Trends in Anal. Chem, 22, 428-439 (2003)
Brunnkvist, H., Karlberg, B., Granelli, I.: Enantiomeric separation of TAPP, H-Tyr–(d)Ala–Phe–Phe–NH2, by capillary electrophoresis using 18-crown-6-tetracarboxylic acid as a chiral selector. J. Chromatogr. B 793, 343–350 (2003)
Ivanyia,T., liaPal,K., Lazar,I., Massart,D.L., Vander Heyden.,Y.,: Application of tetraoxadiaza-crown ether derivatives as chiral selector modifiers in capillary electrophoresis, J. Chromatogr. A, 1028,325–332 (2004)
Yang Gong,X., Hauser,P.C., Enantiomeric separation of 1-phenylethylamine and 1-cyclohexylethylamine in capillary electrophoresis with contactless conductivity detection, J. Chromatography A, 1094,196–199(2005)
Kuhn, R.,: Enantiomeric separation by capillary electrophoresis using a crown ether as chiral selector. Electrophoresis, 20, 2605–2613 (1999)
Kuhn, R., Erni, F., Bereuter, T., Hausler, J.: Chiral recognition and enantiomeric resolution based on host-guest complexation with crown ethers in capillary zone electrophoresis. Anal. Chem. 64, 2815–2820 (1992)
Lin, J.M., Nakagama, T., Hobo, T.: Combined chiral crown ether and β-cyclodextrin for the separation of o-, m-, and p-fluoro-d, l-phenylalanine by capillary gel electrophoresis. Chromatographia 42, 559–565 (1996)
Cho, S.I., Jung, H., Chung, S.: Competitive binding of a Tris run buffer with chiral crown ether in chiral capillary electrophoresis. Electrophoresis 21, 3618–3624 (2000)
Jang, J., Cho, S.I., Chung, D.S.: Comparative studies of various run buffers for chiral capillary electrophoresis using chiral crown ether as a chiral selector. Electrophoresis 22, 4362–4367 (2001)
Kim, E., Koo, Y.-M., Chung, D.S.: Chiral counter-current chromatography of gemifloxacin guided by capillary electrophoresis using (+)-(18-crown-6)-tetracarboxylic acid as a chiral selectorJ. Chromatogr. A 1045, 119–124 (2004)
Pereira, E.A., Tavares, M.F.M.: Determination of volatile corrosion inhibitors by capillary electrophoresis. J. Chromatogr. A 1051, 303–308 (2004)
Chiou, Ch.S., Shih, J-Sh.,: Application of crown ethers as modifiers for the separation of amines by capillary electrophoresis. AnalChimActa 360, 69-76 (1998)
Cho,S., Shimb,J.,Kima,M-S.,Kima,Y-K.,Chung,D.S.,: On-line sample cleanup and chiral separation of gemifloxacin in a urinary solution using chiral crown ether as a chiral selector in microchip electrophoresis, J.Chromatogr. A, 1055, 241–245 (2004)
Thanh Ha, P.T., Hoogmartens, J., Schepdael, A.V.: Recent advances in pharmaceutical applications of chiral capillary electrophoresis. J. Pharm. Biomed. Analysis 41, 1–11 (2006)
Forlay-Frick, P., Mangelings, D., Ivanyi, T., Lazar, I., Heberger, K., Heyden, Y.V.: Newly synthesized tetraoxa-diaza crown ether derivatives versus commercialized crown ethers in the separation of positional isomers with capillary electrophoresis. J. Pharm. Biomed. Analysis 41, 1164–1170 (2006)
Doyle, J.- M., McCord, B.-R.: Novel electrolyte for the analysis of cations in low explosive residue by capillary electrophoresis. J. Chromatogr. B. 714, 105–111 (1998)
Yang, Q., SmeyersVerbeke, J., Wu, W., Khots, M.S., Massart, D.L.: Simultaneous separation of ammonium and alkali, alkaline earth and transition metal ions in aqueous-organic media by capillary ion analysis. J. Chromatogr. A 688, 339–349 (1994)
Beck, W., Engelhardt, H.: Separation of non UV-absorbing cations by capillary electrophoresis. Fresen. J. Anal. Chem. 346, 618–621 (1993)
Lawrence, J.F.: Organic Trace Analysis by Liquid Chromatography. Academic Press, New York (1981)
Riviello, J.M., Harrold, M.P.: Capillary electrophoresis of inorganic cations and low-molecular-mass amines using a copper-based electrolyte with indirect UV detection. J. Chromatogr. A 652, 385–392 (1993)
Fung, Y.S., Lau, K.M., Tung, H.Sh: Analysis of leachable and total trace metals in air particulate matters by capillary electrophoresis. Talanta 45, 619–625 (1998)
Colombara1, R., Massaro, S., Tavares, M. F.M.: Exploring the versatility of capillary electrophoresis for the analysis of ionic species in vehicular emission. Anal. Chim. Acta 388, 171-180 (1999)
Tagliaro, F., Manetto, G., Cittadini, F., Marchetti, D., Bortolotti, F., Marigo, M.: Capillary zone electrophoresis of potassium in human vitreous humour: validation of a new method. J. Chromatogr. B 733, 273–279 (1999)
Yang, Q., Zhuang, Y., Verbeke, J.S., Massart, D.L.: Interpretation of migration behaviour of inorganic cations in capillary ion electrophoresis based on an equilibrium model. J. Chromatogr. A 706, 503–515 (1995)
Francois, C., Morn, Ph, Dreux, M.: Effect of the concentration of 18-crown-6 added to the electrolyte upon the separation of ammonium, alkali and alkaline-earth cations by capillary electrophoresis. J. Chromatogr. A 706, 535–553 (1995)
Francois, C., Morn, Ph, Dreux, M.: Separation of transition metal cations by capillary electrophoresis optimization of complexing agent concentrations (lactic acid and 18-crown-6). J. Chromatogr. A 717, 393–408 (1995)
Lee, Y.-H., Lin, T.-I.: Determination of metal cations by capillary electrophoresis effect of background carrier and completing agents. J. Chromatogr. A 675, 227–236 (1994)
Shi, Y., Fritz, J.S.: New electrolyte systems for the determination of metal cations by capillary zone electrophoresis. J. Chromatogr. A 671, 429–435 (1994)
Oehrle, S.A.: Controlled changes in selectivity of cation separations by capillary electrophoresis using various crown-ether additives. J. Chromatogr. A 745, 87–92 (1996)
Lucy, C.A.: Factors affecting selectivity of inorganic anions in capillary electrophoresis. Journal of Chromatogr. A. 850, 319–337 (1999)
Lamb, J.D., Edwards, B.R., Smith, R.G., Garrick, R.: The use of macrocycles as electroosmotic flow modifiers in the separation of inorganic anions by capillary electrophoresis. Talanta 42, 109–117 (1995)
Okada, T.: Polyethers in inorganic capillary electrophoresis. Journal of Chromatogr.A. 834, 73–87 (1999)
Dabek-Zlotorzynska, E., Dlouhy, J.F.: Application of capillary electrophoresis in atmospheric aerosol analysis: determination of cations. J. Chromatogr. A 706, 527–534 (1995)
Simunicova, E., Kaniansky, D., Loksıkova, K.: Separation of alkali and alkaline earth metal and ammonium cations by capillary zone electrophoresis with indirect UV absorbance detection. J. Chromatogr.A. 665, 203-209 (1994)
Xu, X., Kok, W.Th, Kraak, J.C., Poppe, H.: Simultaneous determination of urinary creatinine, calcium and other inorganic cations by capillary zone electrophoresis with indirect ultraviolet detection. J. Chromatogr. B 661, 35–45 (1994)
Bachmann, K., Boden, J., Haumann, I.: Indirect fluorimetric detection of alkali and alkaline earth metal ions in capillary zone electrophoresis with cerium (III) as carrier electrolyte. J. Chromatogr. 626, 259 (1992)
Yang, Q., Hartmann, C., Smeyers-Verbeke, J., Massart, D.L.: Method development and validation for the determination of mineral elements in food and botanical materials by capillary electrophoresis. J. Chromatogr. A 717, 415–425 (1995)
Fukushi, K., Hiiro, K.: Use of crown ethers in the isotachophoretic determination of metal ions. J. Chromatogr. 523, 281–292 (1990)
Chiou, C.-S., Shih, J.-S.: Bifunctional crypt and modifier for capillary electrophoresis in separations of inorganic/organic anions and inorganic cations. Analyst 121, 1107–1110 (1996)
Stathakis, C., Cassidy, R.M.: Capillary electrophoretic separation of metal ions in the presence of polyethylene glycols. Analyst 121, 839–843 (1996)
Ito, K., Hirokawa, T.: Separation of alkali and alkaline-earth metal and ammonium cations by capillary electrophoresis using poly(ethylene glycol) and tartaric acid. J. Chromatogr. A 742, 281–288 (1996)
Hiissa, T., Siren, H., Kotiaho, T., Snellman, M., Hautojarvi, A.: Quantification of anions and cations in environmental water samples, Measurements with capillary electrophoresis and indirect-UV detection. J. Chromatogr. A 853, 403–411 (1999)
Tagliaro, F., Bortolotti, F., Manetto, G., Cittadini, F.: Potassium concentration differences in the vitreous humour from the two eyes revisited by microanalysis with capillary electrophoresis. J. Chromatogr. A 924, 493–498 (2001)
Pascali, V.L., Marigo, M., Mori, M., Tsue, H., Tanaka, Sh: Cationic diaza crown ether for the separation of positional isomers by capillary electrophoresis. J. Chromatogr. A 922, 399–403 (2001)
Kokkonen, R., Sirén, H., Kauliomäki, S., Rovio, S., Luomanper, K.: On-line process monitoring of water-soluble ions in pulp and paper machine waters by capillary electrophoresis. J. Chromatogr. A 1032, 243–252 (2004)
Rovio, S., Mantynen, M., Siren, H.: Determination of bromide and potassium in saline ground waters by capillary electrophoresis without prior dilution. Appl. Geochem. 19, 1331–1337 (2004)
Hopper, K.G., LeClair, H., McCord, B.R.: A novel method for analysis of explosives residue by simultaneous detection of anions and cations via capillary zone electrophoresis. Talanta 67, 304–312 (2005)
Masara, M., Sydesb, D., Luca, M., Kanianskya, D., Kuss, H.M.: Determination of ammonium, calcium, magnesium, potassium and sodium in drinking waters by capillary zone electrophoresis on a column-coupling chip. J. Chromatogr. A 1216, 6252–6255 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadzadeh kakhki, R., Assadi, H. Capillary electrophoresis analysis based on crown ethers. J Incl Phenom Macrocycl Chem 81, 1–12 (2015). https://doi.org/10.1007/s10847-014-0419-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-014-0419-1