Abstract
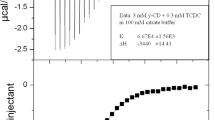
The formation of inclusion complexes of hydroxypropylated β-cyclodextrins (CDs) with three bile salts are investigated to shed light on the role played by the hydroxypropyl (HP) substituents. The HP-chains are situated at the rim of the CD and may thus extend the hydrophobic cavity of the CD. Calorimetric titrations in a broad temperature range and molecular dynamics simulations confirm previous speculations that the HP-chains cause an increase in dehydrated nonpolar surface area upon formation of the complexes. This additional burial of nonpolar surface area, 12–16 Å2 per HP-chain according to the MD simulations, results in more negative values of ΔC p °, which are in quantitative agreement with what is expected for hydrophobic dehydration. Although these observations support the picture of an extended hydrophobic cavity, HPβCD complexes were less stable than their unsubstituted counterparts. This indicates that increased hydrophobic contacts are not always accompanied by increased binding strength. The linear dependence of ΔC p °, ΔH° and ΔS° on the number of HP-chains give rise to isoentropic and isoenthalpic temperatures at which ΔH° and ΔS° are independent of the number of HP-chains on the host CD (but depend on the type of bile salt). Interestingly, these convergence temperatures are close to what is observed for unfolding of proteins and may be a common feature of hydrophobic dehydration.









Similar content being viewed by others
References
Szejtli, J.: Cyclodextrin Technology. Kluwer Academic Publishers, Dordrecht (1988)
Szejtli, J.: Chemistry, physical and biological properties of cyclodextrins. In: Szejtli, J., Osa, T. (eds.) Cyclodextrins, pp. 189–204. Elsevier Science Ltd., Oxford (1996)
Rekharsky, M.V., Inoue, Y.: Complexation thermodynamics of cyclodextrins. Chem. Rev. 98, 1875–1917 (1998)
Dodziuk, H.: Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications. Wiley-VCH, Weinheim (2006)
Uekama, K.: Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. 52, 900–915 (2004)
Uekama, K., Hirayama, F., Irie, T.: Cyclodextrin drug carrier systems. Chem. Rev. 98, 2045–2076 (1998)
Maas, J., Kamm, W.H.G.: An integrated early formulation strategy—from hit evaluation to preclinical candidate profiling. Eur. J. Pharm. Sci. 66, 1–10 (2007)
Neervannan, S.: Preclinical formulation for discovery and toxicology: physicochemical challenges. Expert Opin. Drug Metab. Toxicol. 2, 715–731 (2006)
Strickley, R.G.: Solubilizing excipients in oral and injectable formulations. Pharm. Res. 21, 201–230 (2004)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007)
Loftsson, T., Brewster, M.E., Masson, M.: Role of cyclodextrins in improving oral drug delivery. Am. J. Drug Deliv. 2, 175–261 (2004)
Castronuovo, G., Niccoli, M., Varriale, L.: Complexation forces in aqueous solution. Calorimetric studies of the association of 2-hydroxypropyl-beta-cyclodextrin with monocarboxylic acids or cycloalkanols. Tetrahedron 63, 7047–7052 (2007)
Veiga, M.D., Merino, M., Cirri, M., Maestrelli, F., Mura, P.: Comparative study on triclosan interactions in solution and in the solid state with natural and chemically modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 53, 77–83 (2005)
Castronuovo, G., Niccoli, M.: The cavity elongation effect. Calorimetric studies of the complexes of long-chain carboxylic acids with methyl-α-cyclodextrin in aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 58, 289–294 (2007)
Zia, V., Rajewski, R.A., Stella, V.J.: Thermodynamics of binding of neutral molecules to sulfobutyl ether β-cyclodextrins (SBE-β-CDs): the effect of total degree of substitution. Pharm. Res. 17, 936–941 (2000)
Blach, P., Landy, D., Fourmentin, S., Surpateanu, G., Bricout, H., Ponchel, A., Hapiot, F., Monflier, E.: Sulfobutyl ether-beta-cyclodextrins: promising supramolecular carriers for aqueous organometallic catalysis. Adv. Synth. Catal. 347, 1301–1307 (2005)
Gomez-Biagi, R.F., Jagt, R.B.C., Nitz, M.: Remarkably stable inclusion complexes with heptakis-[6-deoxy-6-(2-aminoethylsulfanyl)]-beta-cyclodextrin. Org. Biomol. Chem. 6, 4622–4626 (2008)
Schönbeck, C., Westh, P., Madsen, J.C., Larsen, K.L., Städe, L.W., Holm, R.: Hydroxypropyl substituted β-cyclodextrins: influence of degree of substitution on the thermodynamics of complexation with tauro- and glyco-conjugated bile salts. Langmuir 26, 17949–17957 (2010)
Myers, J.K., Pace, C.N., Scholtz, J.M.: Denaturant M-values and heat-capacity changes—relation to changes in accessible surface-areas of protein unfolding. Protein Sci. 4, 2138–2148 (1995)
Spolar, R.S., Livingstone, J.R., Record, M.T.: Use of liquid-hydrocarbon and amide transfer data to estimate contributions to thermodynamic functions of protein folding from the removal of nonpolar and polar surface from water. Biochemistry 31, 3947–3955 (1992)
Garidel, P., Hildebrand, A., Neubert, R., Blume, A.: Thermodynamic characterization of bile salt aggregation as a function of temperature and ionic strength using isothermal titration calorimetry. Langmuir 16, 5267–5275 (2000)
Ross, P.D., Rekharsky, M.V.: Thermodynamics of hydrogen bond and hydrophobic interactions in cyclodextrin complexes. Biophys. J. 71, 2144–2154 (1996)
Olvera, A., Perez-Casas, S., Costas, M.: Heat capacity contributions to the formation of inclusion complexes. J. Phys. Chem. B 111, 11497–11505 (2007)
Holm, R., Shi, W., Hartvig, R.A., Askjær, S., Madsen, J.C., Westh, P.: Thermodynamics and structure of inclusion compounds of tauro- and glyco-conjugated bile salts and β-cyclodextrins. Phys. Chem. Chem. Phys. 11, 5070–5078 (2009)
Roda, A., Hofmann, A.F., Mysels, K.J.: The influence of bile salt structure on self-association in aqueous solutions. J. Biol. Chem. 258, 6362–6370 (1983)
Schönbeck, C., Holm, R., Westh, P.: Higher order inclusion complexes and secondary interactions studied by global analysis of calorimetric titrations. Anal. Chem. 84, 2305–2312 (2012)
Humphrey, W., Dalke, A., Schulten, K.: VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996)
bcdtest.inp file in the CHARMM package: Chemistry at Harvard macromolecular mechanics (CHARMM)—Developmental Version 31b2 (2005). (http://www.charmm.org/package/releases.html). Accessed 15 Feb 2005
Pan, Y.H., Bahnson, B.J.: Structural basis for bile salt inhibition of pancreatic phospholipase A2. J. Mol. Biol. 369, 439–450 (2007)
Chatterjee, S., Zhong, D.L., Nordhues, B.A., Battaile, K.P., Lovell, S., De Guzman, R.N.: The crystal structures of the Salmonella type III secretion system tip protein SipD in complex with deoxycholate and chenodeoxycholate. Protein Sci. 20, 75–86 (2011)
Phillips, J.C., Braun, R., Wang, W., Gumbart, J., Tajkhorshid, E., Villa, E., Chipot, C., Skeel, R.D., Kale, L., Schulten, K.: Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005)
MacKerell, A.D., Bashford, D., Bellott, M., Dunbrack, R.L., Evanseck, J.D., Field, M.J., Fischer, S., Gao, J., Guo, H., Ha, S., Joseph-McCarthy, D., Kuchnir, L., Kuczera, K., Lau, F.T.K., Mattos, C., Michnick, S., Ngo, T., Nguyen, D.T., Prodhom, B., Reiher, W.E., Roux, B., Schlenkrich, M., Smith, J.C., Stote, R., Straub, J., Watanabe, M., Wiorkiewicz-Kuczera, J., Yin, D., Karplus, M.: All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998)
Vorobyov, I., Anisimov, V.M., Greene, S., Venable, R.M., Moser, A., Pastor, R.W., MacKerell, A.D.: Additive and classical drude polarizable force fields for linear and cyclic ethers. J. Chem. Theory Comput. 3, 1120–1133 (2007)
Feller, S.E., Zhang, Y.H., Pastor, R.W., Brooks, B.R.: Constant-pressure molecular-dynamics simulation—the Langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995)
Darden, T., York, D., Pedersen, L.: Particle mesh Ewald—an N. Log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993)
Essmann, U., Perera, L., Berkowitz, M.L., Darden, T., Lee, H., Pedersen, L.G.: A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995)
Cabrer, P.R., Alvarez-Parrilla, E., Al-Soufi, W., Meijide, F., Núñez, E.R., Tato, J.V.: Complexation of bile salts by natural cyclodextrins. Supramol. Chem. 15, 33–43 (2003)
Mucci, A., Schenetti, L., Vandelli, M.A., Ruozi, B., Salvioli, G., Forni, F.: Comparison between Roesy and 13C NMR complexation shifts in deriving the geometry of inclusion compounds: a study on the interaction between hyodeoxycholic acid and 2-hydroxypropyl-β-cyclodextrin. Supramol. Chem. 12, 427–433 (2001)
Schönbeck, C., Westh, P., Madsen, J.C., Larsen, K.L., Stade, L.W., Holm, R.: Methylated beta-cyclodextrins: influence of degree and pattern of substitution on the thermodynamics of complexation with tauro- and glyco-conjugated bile salts. Langmuir 27, 5832–5841 (2011)
Holm, R., Madsen, J.C., Shi, W., Larsen, K.L., Städe, L.W., Westh, P.: Thermodynamics of complexation of tauro- and glyco-conjugated bile salts with two modified β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 69, 201–211 (2011)
Harata, K., Rao, C.T., Pitha, J., Fukunaga, K., Uekama, K.: Crystal-structure of 2-O-[(S)-2-hydroxypropyl]cyclomaltoheptaose. Carbohydr. Res. 222, 37–45 (1991)
Harata, K., Rao, C.T., Pitha, J.: Crystal-structure of 6–0-[(R)-2-hydroxypropyl]cyclomaltoheptaose and 6–0-[(S)-2-hydroxypropyl]cyclomaltoheptaose. Carbohydr. Res. 247, 83–98 (1993)
Clarke, E.C.W., Glew, D.N.: Evaluation of thermodynamic functions from equilibrium constants. Trans. Faraday Soc. 62, 539–547 (1966)
Bates, D.M., Watts, D.G.: Nonlinear Regression Analysis and Its Applications. Wiley, New York (2007)
Tan, Z.J., Zhu, X.X., Brown, G.R.: Formation of inclusion complexes of cyclodextrins with bile salt anions as determined by NMR titration studies. Langmuir 10, 1034–1039 (1994)
Pitha, J., Rao, C.T., Lindberg, B., Seffers, P.: Distribution of substituents in 2-hydroxypropyl ethers of cyclomaltoheptaose. Carbohydr. Res. 200, 429–435 (1990)
Rao, C.T., Pitha, J., Lindberg, B., Lindberg, J.: Distribution of substituents in O-(2-hydroxypropyl) derivatives of cyclomalto-oligosaccharides (cyclodextrins): influence of increasing substitution, of the base use in the preparation, and of macrocyclic size. Carbohydr. Res. 223, 99–107 (1992)
Naghibi, H., Dec, S.F., Gill, S.J.: Heats of solution of ethane and propane in water from 0°C to 50°C. J. Phys. Chem. 91, 245–248 (1987)
Cameron, D.L., Jakus, J., Pauleta, S.R., Pettigrew, G.W., Cooper, A.: Pressure perturbation calorimetry and the thermodynamics of noncovalent interactions in water: comparison of protein–protein, protein–ligand, and cyclodextrin–adamantane complexes. J. Phys. Chem. B 114, 16228–16235 (2010)
Cabani, S., Gianni, P., Mollica, V., Lepori, L.: Group contributions to the thermodynamic properties of non-ionic organic solutes in dilute aqueous-solution. J. Solut. Chem. 10, 563–595 (1981)
Gallicchio, E., Kubo, M.M., Levy, R.M.: Enthalpy–entropy and cavity decomposition of alkane hydration free energies: numerical results and implications for theories of hydrophobic solvation. J. Phys. Chem. B 104, 6271–6285 (2000)
Heerklotz, H., Epand, R.M.: The enthalpy of acyl chain packing and the apparent water-accessible apolar surface area of phospholipids. Biophys. J. 80, 271–279 (2001)
Costas, M., Kronberg, B., Silveston, R.: General thermodynamic analysis of the dissolution of nonpolar molecules into water—origin of hydrophobicity. J. Chem. Soc. Faraday Trans. 90, 1513–1522 (1994)
Connelly, P.R., Thomson, J.A.: Heat-capacity changes and hydrophobic interactions in the binding of Fk506 and rapamycin to the Fk506 binding-protein. Proc. Natl. Acad. Sci. USA 89, 4781–4785 (1992)
Zangi, R., Berne, B.J.: Temperature dependence of dimerization and dewetting of large-scale hydrophobes: a molecular dynamics study. J. Phys. Chem. B 112, 8634–8644 (2008)
Plyasunov, A.V., Shock, E.L.: Thermodynamic functions of hydration of hydrocarbons at 298.15 K and 0.1 MPa. Geochim. Cosmochim. Acta 64, 439–468 (2000)
Chandler, D.: Interfaces and the driving force of hydrophobic assembly. Nature 437, 640–647 (2005)
Murphy, K.P., Privalov, P.L., Gill, S.J.: Common features of protein unfolding and dissolution of hydrophobic compounds. Science 247, 559–561 (1990)
Privalov, P.L.: Stability of proteins small globular proteins. In: Anfinsen, C.B. (ed.) Advances in Protein Chemistry, pp. 167–241. Academic Press, New York (1979)
Baldwin, R.L.: Temperature-dependence of the hydrophobic interaction in protein folding. Proc. Natl. Acad. Sci. USA 83, 8069–8072 (1986)
Lee, B.: Isoenthalpic and isoentropic temperatures and the thermodynamics of protein denaturation. Proc. Natl. Acad. Sci. USA 88, 5154–5158 (1991)
Murphy, K.P.: Hydration and convergence temperatures—on the use and interpretation of correlation plots. Biophys. Chem. 51, 311–326 (1994)
Liu, L., Yang, C., Guo, Q.X.: A study on the enthalpy–entropy compensation in protein unfolding. Biophys. Chem. 84, 239–251 (2000)
Acknowledgments
Thanks to the Danish Center for Scientific Computing (DCSC) at the University of Southern Denmark for granting access to their computing resources.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schönbeck, C., Holm, R., Westh, P. et al. Extending the hydrophobic cavity of β-cyclodextrin results in more negative heat capacity changes but reduced binding affinities. J Incl Phenom Macrocycl Chem 78, 351–361 (2014). https://doi.org/10.1007/s10847-013-0305-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-013-0305-2