Abstract
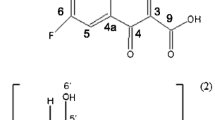
The aim of this study was to investigate the interactions of triclosan (TRI), a poorly water-soluble antimicrobial drug, with natural crystalline cyclodextrins (α-, β- and γ-Cd) and the corresponding hydroxypropylated amorphous derivatives (HPα-, HPβ-, and HPγ-Cd) and evaluate their effectiveness as complexing and solubilizing agents towards the drug. Equimolar solid systems were prepared using different techniques (physical mixing (PM), kneading (KN) and coevaporation (COE)) in order to evaluate the influence of the preparation method on the performance of the end products. Drug–carrier interactions were investigated both in aqueous solution, using phase-solubility analysis, fluorescence and circular dichroism (CD) techniques, and in the solid state, using differential scanning calorimetry (DSC) supported by thermograumetric analysis (TGA), X-ray powder diffractometry (XRPD) and scanning electron microscopy (SEM) analysis. Among the native cyclodextrins, β-Cd seemed to have the most suitable cavity to fit the drug molecule, whereas the α-Cd cavity was too small and the γ-Cd cavity too large to establish stable interactions with the guest. However, due to the B S -type phase solubility diagram, its solubilizing efficiency was very limited. The presence of the hydroxypropylic substituents improved, in all cases, Cd solubilizing and complexing efficacies towards the drug. This was particularly evident in the case of HPγ-Cd, whose stability constant was about 200-fold higher than that of the native γ-Cd. HPβ-Cd was the most effective carrier for TRI, showing a solubilizing power about 20 times higher than the corresponding native Cd and about 2-fold that of the other hydroxypropyl derivatives. Moreover, a clear influence of the preparation method on the properties of the final products was observed. The COE method with hydroxypropylated cyclodextrins seemed the most suitable technique in achieving the complete drug amorphization and/or inclusion complexation.
Similar content being viewed by others
References
J. Fuls G. Fischler (2004) Am. J. Infect. Control 32 3 E22 Occurrence Handle10.1016/j.ajic.2004.04.032
J. Menoutis, PhD. F.A.I.C., C.P.C. and A.I. Parisi. Quantex Laboratories, 2001.
R.J. Gilbert P.E.O. Williams (1987) Brit. J. Clin. Pharmacol. 23 579
M. Addy S. Jenkins R. Newcombe (1989) Am. J. Dent. 2 215 Occurrence Handle2638182
D. Cummins J.E. Creeth (1992) J. Dent. Res. 71 1439 Occurrence Handle1629461
T. Loftsson N. Leeves B. Bjornsdottir L. Duffy M. Masson (1999) J. Pharm. Sci. 88 1254 Occurrence Handle10.1021/js9902466 Occurrence Handle10585219
C. Grove W. Liebenberg J.L. Du Preez W. Yang M.M. De Villiers (2003) J. Cosmet. Sci. 54 537 Occurrence Handle14730370
Duchêne D. ▮. In De Santè (ed.), Cyclodextrin and Their Industrial Uses, ▮,Paris (1987), pp. 448–481.
J. Szejtli (1988) Cyclodextrin Technology Kluwer Academic Publishers Dordrecht 75
K. Uekama F. Hirayama T. Irie (1998) Chem. Rev. 98 2045 Occurrence Handle10.1021/cr970025p Occurrence Handle11848959
F. Melani G.P. Bettinetti P. Mura A. Manderioli (1995) J. Inclusion Phenom. 22 131 Occurrence Handle10.1007/BF00707688
P. Mura E. Adragna A.M. Rabasco J.R. Moyano J.I. Pérez- Martìnez M.J. Arias J.M. Ginés (1999) Drug. Dev. Ind. Pharm. 25 279 Occurrence Handle10.1081/DDC-100102172 Occurrence Handle10071820
P. Mura G.P. Bettinetti F. Melani A. Manderioli (1995) Eur J. Pharm. Sci. 3 347 Occurrence Handle10.1016/0928-0987(95)00025-X
G.P. Bettinetti P. Mura M.T. Faucci M. Sorrenti M. Setti (2002) Eur. J. Pharm Sci. 15 21 Occurrence Handle10.1016/S0928-0987(01)00199-3 Occurrence Handle11803128
P. Mura F. Maestrelli M. Cirri S. Furlanetto S. Pinzauti (2003) Drug. Dev. Ind. Pharm. 73 635
T. Higuchi K. Connors (1965) Adv. Anal. Chem. Instrum. 4 117
A. Yoshida M. Yamamoto T. Irie F. Hirayama K. Uekama (1989) Chem. Pharm. Bull. 37 1059 Occurrence Handle2766408
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in final form: 24 January 2005
Rights and permissions
About this article
Cite this article
Veiga, M.D., Merino, M., Cirri, M. et al. Comparative Study on Triclosan Interactions in Solution and in the Solid State with Natural and Chemically Modified Cyclodextrins. J Incl Phenom Macrocycl Chem 53, 77–83 (2005). https://doi.org/10.1007/s10847-005-1047-6
Issue Date:
DOI: https://doi.org/10.1007/s10847-005-1047-6