Abstract
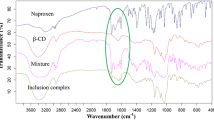
Inclusion complex formation between β-cyclodextrin and Naproxen was investigated using differential scanning calorimetry (DSC) as a function of the β-cyclodextrin-to-Naproxen molar ratio, ranging from 0:5:1 to 5:1. When these mixtures are heated above the melting temperature of Naproxen, an exothermic peak is observed at a temperature slightly higher than the melting peak of Naproxen. This peak, which has not been previously reported, has been interpreted as an exothermic energy of inclusion complex formation. The magnitude of this complex formation peak was found to be dependent upon the composition of the β-cyclodextrin and Naproxen mixture and increased in magnitude to a maximum value at a β-cyclodextrin:Naproxen molar ratio of 2:1. In addition, Naproxen recrystallization and re-melting peaks seen in the cooling and re-heating scans, respectively, decreased in magnitude with increasing molar ratio and totally disappeared for the mixture with 5:1 of β-cyclodextrin to Naproxen ratio indicative of complete inclusion of Naproxen in the cyclodextrin cavities. Complete inclusion was further reflected by the disappearance of key Naproxen peaks in Fourier transform infrared spectra of samples recovered from DSC experiments. The large excess of β-cyclodextrin needed to fully complex the Naproxen was found to be due to slow kinetics. Increasing the hold time after the initial melting led to inclusion efficiencies up to 95 % even for the 2:1 mixture. These experiments suggest that ratios of β-cyclodextrin:Naproxen 2:1 or greater facilitate the process by increasing the presence of cyclodextrin molecules in the close proximity of the drug molecules and lead to high inclusion efficiencies.











Similar content being viewed by others
References
Junquera, E., Aicart, E.: A fluorimetric, potentiometric and conductimetric study of the aqueous solutions of Naproxen and its association with hydroxypropyl-beta-cyclodextrin. Int. J. Pharm. 176(2), 169–178 (1999)
Lombardino, J.G.: Nonsteroidal antiinflammatory drugs. Chemistry and pharmacology of drugs, vol. 5. Wiley, New York (1985)
Kawabata, A.: Prostaglandin E(2) and pain-an update. Biol. Pharm. Bull. 34(8), 1170–1173 (2011)
Carrier, R.L., Miller, L.A., Ahmed, I.: The utility of cyclodextrins for enhancing oral bioavailability. J Control Release 123(2), 78–99 (2007)
Challa, R., Ahuja, A., Ali, J., Khar, R.K.: Cyclodextrins in drug delivery: an updated review. Aaps Pharmscitech 6(2), E329–E357 (2005)
Martin Del Valle, E.M.: Cyclodextrins and their uses: a review. Process. Biochem. 39(9), 1033–1046 (2004)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. USA 85(10), 1017–1025 (1996)
Loftsson, T., Duchene, D.: Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 329(1–2), 1–11 (2007)
Bender, M.L., Komiyama, M.: Cyclodextrin Chemistry. Reactivity and Structure, vol. 6. Springer, Berlin (1978)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins: basic science and product development. J. Pharm. Pharmacol. 62(11), 1607–1621 (2010)
Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications. Wiley, Hoboken (2011)
Sadlej-Sosnowska, N., Kozerski, L., Bednarek, E., Sitkowski, J.: Fluorometric and NMR studies of the naproxen-cyclodextrin inclusion complexes in aqueous solutions. J. Incl. Phenom. Macro. 37(1–4), 383–394 (2000)
Bettinetti, G., Sorrenti, M., Negri, A., Setti, M., Mura, P., Melani, F.: Interaction of Naproxen with alpha-cyclodextrin and its noncyclic analog maltohexaose. Pharmaceut. Res. 16(5), 689–694 (1999)
Wang, J., Warner, I.M.: Studies of the naproxen–beta-cyclodextrin inclusion complex. Microchem. J. 48(2), 229–239 (1993)
Bettinetti, G., Mura, P., Faucci, M.T., Sorrenti, M., Setti, M.: Interaction of Naproxen with noncrystalline acetyl beta- and acetyl gamma-cyclodextrins in the solid and liquid state. Eur. J. Pharm. Sci. 15(1), 21–29 (2002)
Bettinetti, G.P., Sorrenti, M., Rossi, S., Ferrari, F., Mura, P., Faucci, M.T.: Assessment of solid-state interactions of Naproxen with amorphous cyclodextrin derivatives by DSC. J. Pharmaceut. Biomed. 30(4), 1173–1179 (2002)
Junco, S., Casimiro, T., Ribeiro, N., Da Ponte, M.N., Marques, H.M.C.: Optimisation of supercritical carbon dioxide systems for complexation of naproxen: beta-cyclodextrin. J. Incl. Phenom. Macro. 44(1–4), 69–73 (2002)
Moribe, K., Fujito, T., Tozuka, Y., Yamamoto, K.: Solubility-dependent complexation of active pharmaceutical ingredients with trimethyl-beta-cyclodextrin under supercritical fluid condition. J. Incl. Phenom. Macro. 57(1–4), 289–295 (2007)
Mura, P., Bettinetti, G.P., Cirri, M., Maestrelli, F., Sorrenti, M., Catenacci, L.: Solid-state characterization and dissolution properties of naproxen-arginine-hydroxypropyl-beta-cyclodextrin ternary system. Eur. J. Pharm. Biopharm. 59(1), 99–106 (2005)
Machin, R., Isasi, J.R., Velaz, I.: Beta-cyclodextrin hydrogels as potential drug delivery systems. Carbohyd. Polym. 87(3), 2024–2030 (2012)
Junco, S., Casimiro, T., Ribeiro, N., Da Ponte, M.N., Marques, H.C.: A comparative study of naproxen—beta cyclodextrin complexes prepared by conventional methods and using supercritical carbon dioxide. J. Incl. Phenom. Macro. 44(1–4), 117–121 (2002)
Banik, A., Gogoi, P., Saikia, M.D.: Interaction of Naproxen with beta-cyclodextrin and its derivatives/polymer: experimental and molecular modeling studies. J. Incl. Phenom. Macro. 72(3–4), 449–458 (2012)
Mura, P., Maestrelli, F., Cirri, M.: Ternary systems of Naproxen with hydroxypropyl-beta-cyclodextrin and aminoacids. Int. J. Pharm. 260(2), 293–302 (2003)
Ganzagonzalez, A., Vilajato, J.L., Anguianoigea, S., Oteroespinar, F.J., Blancomendez, J.: A proton nuclear-magnetic-resonance study of the inclusion complex of Naproxen with beta-cyclodextrin. Int. J. Pharm. 106(3), 179–185 (1994)
Arancibia, J.A., Escandar, G.M.: Determination of Naproxen in pharmaceutical preparations by room-temperature phosphorescence. A comparative study of several organized media. Analyst 126(6), 917–922 (2001)
Kurkov, S.V., Ukhatskaya, E.V., Loftsson, T.: Drug/cyclodextrin: beyond inclusion complexation. J. Incl. Phenom. Macro. 69(3–4), 297–301 (2011)
Gines, J.M., Arias, M.J., Perez-Martinez, J.I., Moyano, J.R., Morillo, E., Sanchez-Soto, P.J.: Determination of the stoichiometry of 2,3-dichlorophenoxyacetic acid beta-cyclodextrin complexes in solution and in solid state. Thermochim. Acta 321(1–2), 53–58 (1998)
Singh, R., Bharti, N., Madan, J., Hiremath, S.: Characterization of cyclodextrin inclusion complexes—a review. J. Pharm. Sci. Technol. 2(3), 171–183 (2010)
Mura, P., Maestrelli, F., Cirri, M., Furlanetto, S., Pinzauti, S.: Differential scanning calorimetry as an analytical tool in the study of drug-cyclodextrin interactions. J. Therm. Anal. Calorim. 73(2), 635–646 (2003)
Al-Marzouqi, A., Jobe, B., Corti, G., Cirri, M., Mura, P.: Physicochemical characterization of drug-cyclodextrin complexes prepared by supercritical carbon dioxide and by conventional techniques. J. Incl. Phenom. Macro. 57(1–4), 223–231 (2007)
Al-Marzouqi, A.H., Elwy, H.M., Shehadi, I., Adem, A.: Physicochemical properties of antifungal drug-cyclodextrin complexes prepared by supercritical carbon dioxide and by conventional techniques. J. Pharmaceut. Biomed. 49(2), 227–233 (2009)
Banchero, M., Manna, L.: The use of lysine to enhance the supercritical complexation of ketoprofen and cyclodextrins. J. Supercrit. Fluids 67, 76–83 (2012)
Koester, L.S., Xavier, C.R., Mayorga, P., Bassani, V.L.: Influence of beta-cyclodextrin complexation on carbamazepine release from hydroxypropyl methylcellulose matrix tablets. Eur. J. Pharm. Biopharm. 55(1), 85–91 (2003)
Spyriouni, T., Krokidis, X., Economou, I.G.: Thermodynamics of pharmaceuticals: prediction of solubility in pure and mixed solvents with PC-SAFT. Fluid Phase Equilibr. 302(1–2), 331–337 (2011)
Dodziuk, H.: Rigidity versus flexibility. A review of experimental and theoretical studies pertaining to the cyclodextrin nonrigidity. J. Mol. Struct. 614(1–3), 33–45 (2002)
Acknowledgments
This research was funded by the National Science Foundation (CBET #0929978).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grandelli, H.E., Stickle, B., Whittington, A. et al. Inclusion complex formation of β-cyclodextrin and Naproxen: a study on exothermic complex formation by differential scanning calorimetry. J Incl Phenom Macrocycl Chem 77, 269–277 (2013). https://doi.org/10.1007/s10847-012-0241-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0241-6