Abstract
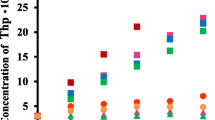
Cyclosophoraoses [cyclic β-(1,2)-glucan, Cys] isolated from Rhizobium leguminosarum biovar trifolii TA-1 have unique structures and high solubility, which make it a potent solubilizer for host–guest inclusion complexation. Succinylated cyclosophorasoses (S-Cys) were also synthesized by chemically modifying isolated cyclosophoraoses. In ultraviolet-visible studies using naproxen (NAP), Cys was shown to form the most stable complexes with NAP (K 1:1 = 2457.9 M−1), which was followed by the negatively charged S-Cys (K 1:1 = 357.1 M−1) at pH 3.4. A further strong reduction in the complex stability constant was observed at pH 7.5. When the reduction in the stability constant was compared with other cyclic oligosaccharides (Cys; 119.2 M−1, CD; 14.48 M−1 and HP-CD; 6.75 M−1), S-Cys (K 1:1 = 5.6 M−1) was shown to have the highest decrease in stability constant. These results suggest that the S-Cys could regulate the efficiency of inclusion complexation at external pH values. NMR studies of complex formation between NAP and Cys also showed a different correlation pattern at pH 3.4 and 7.5. This difference in correlation demonstrates that the inclusion complexes between Cys and NAP formed as a result of the differential charge distribution of the carboxyl groups of NAP. The pH-dependent inclusion behavior of Cys for NAP was also evaluated using molecular docking simulations.







Similar content being viewed by others
Abbreviations
- Cys:
-
Cyclosophoraoses
- S-Cys:
-
Succinlyated cyclsophoraoses
- NAP:
-
Naproxen
- CD:
-
β-Cyclodextrin
- HP-CD:
-
Hydroxypropyl-β-cyclodextrin
- MALDI-TOF:
-
Matrix assisted laser desorption/ionization-time of flight
- NMR:
-
Nuclear magnetic resonance
- DSC:
-
Differential scanning calorimetry
- ROESY:
-
Rotating frame nuclear overhauser effect spectroscopy
- DP:
-
Degree of polymerization
- TLC:
-
Thin layer chromatography
- DS:
-
Degree of substitution
- Deuterium oxide:
-
D2O
- Deuterium methanol:
-
CD3OD
- DEAE:
-
Diethylaminoethyl
- FID:
-
Free induction decay
References
Abe, M., Amemura, A., Higashi, S.: Studies on cyclic beta-l,2-glucan obtained from periplasmic space of Rhizobium trifolii cells. Plant Soil 64(3), 315–324 (1982)
Amemura, A., Hisamatsu, M., Mitani, H., Harada, T.: Cyclic (1 → 2)-b-d-glucan and the octasaccharide repeating-units of extracellular acidic polysaccharides produced by Rhizobium. Carbohydr. Res. 114, 277–285 (1983)
Breedveld, M.W., Zevenhuizen, L.P.T.M., Zehnder, A.J.B.: Excessive excretion of cyclic beta-(1,2)-glucan by Rhizobium trifolii TA-1. Appl. Environ. Microbiol. 56(7), 2080–2086 (1990)
Andre’, L., Mazeau, K., Taravel, F.R., Tvaroska, I.: Conformation and dynamics of a cyclic (1 → 2)-beta-d-glucan. Int. J. Biol. Macromol. 17(3–4), 189–198 (1995)
Spaink, H.P.: Rhizobial lipo-oligosaccharides: answers and questions. Plant Mol. Biol. 20(5), 977–986 (1992)
Zorreguieta, A., Cavaignac, S., Geremia, R.A., Ugalde, R.A.: Osmotic regulation of beta(1–2) glucan synthesis in members of the family Rhizobiaceae. J. Bacteriol. 172(8), 4701–4704 (1990)
Leighand, J.L., Coplin, D.L.: Exopolysaccharides in plant-bacterial interactions. Annu. Rev. Microbiol. 46, 307–346 (1992)
Miller, K.J., Kennedy, E.P., Reinhold, V.N.: Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science 231(4733), 48–51 (1986)
Koizumi, K., Okada, Y., Horiyama, S., Utamura, T., Higashiura, T., Ikeda, M.: Preparation of cyclosophoraose-A and its complex-forming ability. J. Incl. Phenom. 2, 891–899 (1984)
Okada, Y., Horiyama, S., Koizumi, K.: Studies on inclusion complexes of non-steroidal anti-inflammatory agents with cyclosophoraose-A. Yakugaku Zasshi 106, 240–247 (1986)
Kwon, C., Choi, Y., Kim, N., Yoo, J., Yang, C., Kim, H., Jung, S.: Complex forming ability of a family of isolated cyclosophoraoses with erogosterol and its Monte Carlo docking comutatational analysis. J. Incl. Phenom. 36, 55–65 (2000)
Lee, S., Kwon, C., Choi, Y., Seo, D., Kim, H., Jung, S.: Inclusion complexation of a family of cyclosophoraoses with indomethacin. J. Microbiol. Biotechnol. 11(3), 463–468 (2001)
Lee, S., Seo, D., Kim, H., Jung, S.: Investigation of inclusion complexation of paclitaxel by cyclohenicosakis-(1 → 2)-(β-d-glucopyranosyl), by cyclic-(1 → 2)-β-d-glucans (cyclo-sophoraoses), and by cyclomaltoheptaoses (β-cyclodextrins). Carbohydr. Res. 334(2), 119–126 (2001)
Morris, V.J.: Biotechnically produced carbohydrates with functional properties for use in food systems. Food Biotechnol. 4, 45–57 (1990)
Morris, V.J., Brownsey, G.J., Chilvers, G.R., Harris, J.E., Gunning, A.P., Stevens, B.H.J.: Possible biological roles for Rhizobium leguminosarum extracellular polysaccharide and cyclic glucans in bacteria-plant interactions for nitrogen-fixing bacteria. Food Hydrocoll. 5, 185–188 (1991)
Lee, S., Park, H., Seo, D., Choi, Y., Jung, S.: Synthesis and characterization of carboxymethylated cyclosophoraose, and its inclusion complexation behavior. Carbohydr. Res. 339(3), 519–527 (2004)
Park, H., Choi, Y., Kang, S., Lee, S., Kwon, C., Jung, S.: pH-dependent inclusion complexation of carboxymethylated cyclosophoraoses to N-acetyl phenylalanine. Carbohydr. Polym. 64, 85–89 (2006)
Jeon, Y., Kwon, C., Cho, E., Jung, S.: Carboxymethylated cyclosophoraose as a novel chiral additive for the stereoisomeric separation of some flavonoids by capillary electrophoresis. Carbohydr. Res. 345(16), 2408–2412 (2010)
Park, H., Lee, S., Kang, S., Jung, Y.J., Jung, S.: Enantioseparation using sulfated cyclosophoraoses as a novel chiral additive in capillary electrophoresis. Electrophoresis 25(16), 2671–2674 (2004)
Kwon, C., Jung, S.: pH-dependent inclusion complexation of highly succinylated cyclosophoraoses with 4′-hydroxyflavanone. Bull. Korean Chem. Soc. 32(8), 2791–2794 (2011)
Mahler, D.L., Forrest, W.H., Brown, C.R., Shroft, P.F., Gordon, H.E., Brown, B.W., James, K.E.: Assay of aspirin and naproxen analgesia. Clin. Pharmacol. Ther. 19(1), 18–22 (1976)
Calvo, M.V., Lanao, J.M., Dominguez-gil, A.: Bioavailability of rectally administered naproxen. Int. J. Pharm. 38, 117–122 (1987)
Sevelius, M.H., Runkel, R., Segre, E., Blomfield, S.S.: Bioavailability of naproxen sodium and its relationship to clinical analgesic effects. Br. J. Clin. Pharmacol. 10(3), 259–263 (1980)
Valero, M., Carrillo, C., Rodríguez, L.J.: Ternary naproxen: beta-cyclodextrin: polyethylene glycol complex formation. Int. J. Pharm. 265(1–2), 141–149 (2003)
Higuchi, T., Connors, K.A.: Phase solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Constantin, M., Fundueanu, G.: Cyclodextrin-containing poly(vinyl alcohol) as non-viral gene delivery systems. 1. Preparation of polymers. Revue Roumaine de Chimie 54, 1031–1039 (2009)
Job, P.: Formation and stability of inorganic complexes in solution. Annali di Chimica Applicata 9, 113–203 (1928)
Forgo, P., D’Souza, V.T.: The application of selective ROE experiments to study solution structures of cyclomaltooligosacharide derivatives and complexes. Carbohydr. Res. 306(4), 473–478 (1998)
Connor, K.A., Mollica, A.J.: Theoretical analysis of comparative studies of complex formation. J. Pharm. Sci. 55(8), 772–780 (1966)
Friesner, R.A., Banks, J.L., Murphy, R.B., Halgren, T.A., Klicic, J.J., Mainz, D.T., Repasky, M.P., Knoll, E.H., Shelley, M., Perry, J.K., Shaw, D.E., Francis, P., Shenkin, P.S.: Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47(7), 1739–1749 (2004)
Kim, H., Jeong, K., Lee, S., Jung, S.: Molecular dynamics simulation of cyclosophoroheptadecaose (Cys-A). J. Comput. Aided Mol. Des. 16(8–9), 601–610 (2002)
Klausen, K.S., Langmyhr, F.J.: The use of the method of continuous variation for the classification of complexes with mole ratio 1:1. Anal. Chim. Acta 28, 335–340 (1963)
Erden, N., Çelebi, N.: A study of the inclusion complex of naproxen with β-cyclodextrin. Int. J. Pharm. 48, 83–89 (1988)
Bettinetti, G., Melani, F., Mura, P., Monnanni, R., Giordano, H.: Carbon-13 nuclear magnetic resonance study of naproxen interaction with cyclodextrins in solution. J. Pharm. Sci. 80(12), 1162–1170 (1991)
Bettinetti, G., Mura, P., Faucci, M.T., Sorrenti, M., Setti, M.: Interaction of naproxen with noncrystalline acetyl beta- and acetyl gamma-cyclodextrins in the solid and liquid state. Eur. J. Pharm. Sci. 15(1), 21–29 (2002)
Kurkov, S.V., Ukhatskaya, E.V., Loftsson, T.: Drug/cyclodextrin: beyond inclusion complexation. J. Incl. Phenom. 69, 297–301 (2011)
Mosmann, T.: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65(1–2), 55–63 (1983)
Hettiarachchi, G., Nguyen, D., Wu, J., Lucas, D., Ma, D., Isaacs, L., Briken, V.: Toxicology and drug delivery by cucurbit[n]uril type molecular containers. PLoS One 5(5), e10514 (2010)
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2011-0026022) and by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093824). SDG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwon, C., Choi, Y., Jeong, D. et al. Inclusion complexation of naproxen with cyclosophoraoses and succinylated cyclosophoraoses in different pH environments. J Incl Phenom Macrocycl Chem 74, 325–333 (2012). https://doi.org/10.1007/s10847-012-0119-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0119-7