Abstract
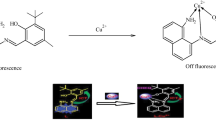
The complexation of thiabendazole (TBZ) with the cucurbit[6]uril (Q[6]), cucurbit[7]uril (Q[7]) and symmetric tetramethyl-cucurbit[6]uril (TMeQ[6]) in aqueous solution has been investigated using UV–vis and fluorespectrometry. The experimental results show 1:1 host–guest inclusion complexes at pH 6.5 for all three macrocyclic hosts, and the corresponding formation constants by UV and fluorescence methods are (5.37 ± 1.05) × 104 L mol−1 and (1.47 ± 0.41) × 104 L mol−1 for the Q[6]-TBZ system (7.76 ± 0.51) × 104 L mol−1 and (9.36 ± 0.22) × 104 L mol−1 for the Q[7]-TBZ system (1.28 ± 0.78) × 104 L mol−1 and (2.69 ± 0.55) × 104 L mol−1 for the TMeQ[6]-TBZ system, respectively. Based on the enhancement of the fluorescence intensity of TBZ with the addition of Q[n]s in neutral media, a fluorespectrometry method for the determination of TBZ in aqueous solution in the presence of Q[n] was established. In the range of 6.0 × 10−8 mol L−1–8.0 × 10−6 mol L−1 a linear relationship was obtained between fluorescence intensity and TBZ concentration. The detection limit was found to be between 5.51 and 8.85 × 10−9 mol L−1. The interference of coexisting ions was found to be slight. The proposed method has been successfully applied to the determination of TBZ in different aqueous solutions with satisfactory recoveries of 92–103%. The method seems to be suitable for environmental water analysis.





Similar content being viewed by others
References
The Compilation of Residues Limits Standards for Pesticides and Veterinary Drugs in Foodstuffs in the World. Dalian Maritime University Press, Dalian (2002)
Muccio, A.D., Girolimetti, S., Barbini, D.A., Pelosi, P., Generali, T., Vergori, L., Merulis, G.D., Leonelli, A., Stefanilli, P.: Selective clean-up applicable to aqueous acetone extracts for the determination of carbendazim and thiabendazole in fruits and vegetables by high-performance liquid chromatography with UV Detection. J. Chromatogr. A 833, 61–65 (1999)
Fernandez-Alba, A.R., Tejedor, A., Aguera, A., Contreras, M., Garrido, J.: Determination of imidacloprid and benzimidazole residues in fruit and vegetables by liquid chromatography-mass spectrometry after ethyl acetate multiresidue extraction. J. AOAC Int. 83, 748–755 (2000)
Liu, X.S., Ton, Z.F., Zheng, L., Huo, H.H., Liu, J.Y.: Simultaneous determination of thiabendazole and carbendazim residues in concentrated pineapple juice by solid phase extraction and high performance liquid chromatography. J. Anal. Sci. 23, 311–314 (2007)
Hu, Y.X., Yang, X.M., Wang, Z., Wang, C., Zhao, J.: Determination of carbendazim and thiabendazole in tomatoes by solid-phase microextraction coupled with high performance liquid chromatography and fluorescence detection. Chin. J. Chromatogr. 23, 81–584 (2005)
Li, J.H., He, Q., Zhao, J., Kong, X.H.: Rapid determination of thiabendazole in juice concentrate by ion exchange chromatography and fluorescence detection. Agrochemicals 46, 772–773 (2007)
Tang, B., Ma, L., Wang, H.Y., Zhang, G.Y.: Study on the supramolecular interation of curcumin and β-cyclodextrin by spectrophotometry and its analytical application. J. Agric. Food Chem. 50, 1355–1361 (2002)
Ma, L., Tang, B., Chu, C.: Spectrofluorimetric study of the β-cyclodextrin-dapsone -linear alcohol supramolecular system and determination of dapsone. Anal. Chim. Acta 469, 183–273 (2002)
Tang, B., Ma, L., Ma, C.: Spectrofluorimetric study of the β-cyclodextrin-rubidate complex and determination of rubidate by β-CD-enhanced fluorimetry. Talanta 58, 841–848 (2002)
Tang, B., Wang, X., Liang, H.L., Jia, B.X., Chen, Z.: Study on the supramolecular interaction of tiabendazole and β-Cyclodextrin by spectrophotometry and is analytical application. J. Agric. Food Chem. 53, 8452–8459 (2005)
Natalia, L.P., Alicia, V.V.: Determination of poorly fluorescent carbamate pesticides in water, bendiocarb and promecarb, using cyclodextrin nanocavities and related media. Anal. Chim. Acta 583, 63–71 (2007)
Natalia, L., Pacioni, V.N., Sueldo, O., Márcio, L., Alicia, V.V.: Spectrofluorimetric determination of benzoimidazolic pesticides: effect of p-sulfonatocalix[6]arene and cyclodextrins. Anal. Chim. Acta 624, 133–140 (2008)
Kim, K., Selvapalam, N., Ko, Y.H., Park, K.M., Kim, D., Kim, J.: Functionalized cucurbiturils and their applications. Chem. Soc. Rev. 36, 267–279 (2007)
Freeman, W.A., Mock, W.L., Shih, N.Y.: Cucurbituril. J. Am. Chem. Soc. 103, 367–7368 (1981)
Day, A.I., Arnold, A.P.: Method for synthesis cucurbiturils[P]. WO 0068232, 8 (2000)
Kim, J., Jung, I.S., Kim, S.Y., Lee, E., Kang, J.K., Sakamoto, S., Yamaguchi, K., Kim, K.: New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7 and 8). Am. Chem. Soc. 122, 540–541 (2000)
Day, A.I., Blanch, R.J., Amold, A.P.: A cucurbituril-based gyroscane: a new supramolecular form. Angew. Chem. Int. Ed. 41, 275–277 (2002)
Blanck, R.J., Sleeman, A.J., White, T.J., Arnold, A.P., Day, A.I.: Cucurbit[7]uril and o-carborane self-assemble to form a molecular ball bearing. Nano. Lett. 2, 147–149 (2002)
Liu, J.X., Tao, Z., Xue, S.F., Zhu, Q.J., Zhang, J.X.: Investigation of host-guest compounds of cucurbit[n = 5–8]uril with some piperazine derivatives. Chin. J. Inorg. Chem. 20, 139–147 (2004)
Lagona, J., Mukhopadhyay, P., Chakrabarti, S., Isaacs, L.: The cucurbit[n]uril family. Angew. Chem. Int. Ed. 44, 4844–4870 (2005)
Li, C.J., Li, J., Jia, X.S.: Selective binding and highly sensitive fluorescent sensor of palmatine and dehydrocorydaline alkaloids by cucurbit[7]uril. Org. Biomol. Chem. 7, 2699–2703 (2009)
Mix, R., Hoffmann, K., Buschmann, H.-J., Friedrich, J.F., Resch-Genger, U.: Coupling of fluorescence labels to plasma-chemically functionalized and cucurbituril modified surfaces. Vakuum in Forschung und Praxis 19, 31–37 (2007)
Zhou, Y.Y., Yu, H.P., Zhang, L., Xu, H.W., Wu, L., Sun, J.Y., Wang, L.: A new spectrofluorometric method for the determination of nicotine base on the inclusion interaction of methylene blue and cucurbit[7]uril. Microchim. Acta. 164, 63–68 (2009)
Zhou, Y.Y., Yu, H.P., Zhang, L., Sun, J.Y., Wu, L., Lu, Q., Wang, L.: Host properties of cucurbit [7] uril: fluorescence enhancement of acridine orange. J. Incl. Phenom. Macrocycl. Chem. 61, 259–264 (2008)
Apurba, L.K., Werner, M.N.: Cucurbituril encapsulation of fluorescent dyes. Supramol. Chem. 19, 55–66 (2007)
Zhou, Y.-Y., Yang, J., Liu, M., Wang, S.-F., Lu, Q.: A novel fluorometric determination of melamine using cucurbit[7]uril. J. Lumin. 130, 817–820 (2010)
Li, C.-F., Dua, L.-M., Wua, W.-Y., Sheng, A.-Z.: Supramolecular interaction of cucurbit[n]urils and coptisine by spectrofluorimetry and its analytical application. Talanta 80, 1939–1944 (2010)
Li, C.-F., Dua, L.-M., Zhang, H.-M.: Study on the inclusion interaction of cucurbit[n]urils with sanguinarine by spectrofluorimetry and its analytical application. Spectrochim. Acta Part A 75, 912–917 (2010)
Baumes, L.A., Sogo, M.B., Montes-Navajas, P., Corma, A., Garcia, H.: A colorimetric sensor array for the detection of the date-rape drug γ-hydroxybutyric acid (GHB): a supramolecular approach. Chem. Eur. J. 16, 4489–4495 (2010)
Saleh, N., Al-Rawashdeh, N.A.F.: Fluorescence enhancement of carbendazim fungicide in cucurbit[6]uril. J. Fluoresc. 16, 487–493 (2006)
Saleh, N., Koner, A.L., Nau, W.M.: Activation and stabilization of drugs by supramolecular pKa shifts: drug-delivery applications tailored for cucurbiturils. Angew. Chem. Int. Ed. 47, 5398–5401 (2008)
Pozo, M.D., Hernández, L., Quintana, C.: A selective spectrofluorimetric method for carbendazim determination in oranges involving inclusion-complex formation with cucurbit[7]uril. Talanta 81, 1542–1546 (2010)
Zhao, Y.J., Xue, S.F., Zhu, Q., Tao, Z., Zhang, J.X., Wei, Z.B., Long, L.S., Hu, M.L., Xiao, H.P., Day, A.I.: Synthesis of a symmetrical tetrasubstituted cucurbit[6]uril and its host-guest compound with 2, 2′-bipyridine. Chin. Sci. Bull. 49, 1111–1116 (2004)
Parker, C.A.: Photoluminescence of solutions with applications to photochemistry and analytical chemistry. Elsevier, Amsterdam (1968)
Demas, J.N., Crosby, G.A.: The measurement of photoluminescence quantum yields. A review. J. Phys. Chem. 75, 991–1024 (1971)
Inczédy, J., Lengyel, T., Ure, A., Gelencsér, A.A.: Compendium of analytical nomenclature (Definitive Rules 1997). Blackwell Science, England (1998)
Patrlcla, C.T., Cllne Love, L.J.: Effects of excited-state prototropic equilibria on the fluorescence energies of benzimidazole and thiabendazole homologues. J. Phys. Chem. 86, 5227–5230 (1982)
Liu, Y., You, C.C., Zhang, H.Y.: Supramolecular chemistry—molecular recognition and assembly of synthesis receptor. Nankai University Press, Tianjin (2001)
Koropchak, J.A., Zlotorzynska, E.D.: Tubular donnan dialysis-inductively coupled plasma atomic emission spectrometry. Anal. Chem. 60, 328–331 (1988)
Capitán, K., Alonso, E.J., Avidad, R., Capitán-Vallvey, L.E., Vilchez, J.L.: Determination of thiabendazole residues in waters by solid-phase spectrofluorometry. Anal. Chem. 65, 1336–1339 (1993)
Dilna, V., Rex, H., Jeff, S., Whitlock, S.A.: Methods for the determination of maleic hydrazide, ethoxyquin and thiabendazole in wastewaters. J. Chromatogr. A 283, 383–389 (1984)
Rekharsky, M.V., Ko, Y.H., Selvapalam, N., Kim, K., Inoue, Y.: Complexation thermodynamics of cucurbit[6]uril with aliphatic alcohols, amines, and diamines. Supramol. Chem. 19, 39–46 (2007)
Montes-Navajas, P., Baumes, L.A., Corma, A., Garcia, H.: Dual-response colorimetric sensor array for the identification of amines in water based on supramolecular host-guest complexation. Tetrahedron Lett. 50, 2301–2304 (2009)
Buschmann, H.J., Mutihac, L., Schollmeyer, E.: The formation of homogeneous and heterogeneous 2:1 complexes between dialkyl- and diarylammonium ions and α-cyclodextrin and cucurbit[6]uril in aqueous formic acid. Thermochim. Acta. 495, 28–32 (2009)
Karcher, S., Kornmueller, A., Jekel, M.: Effects of alkali and alkaline-earth cations on the removal of reactive dyes with cucurbituril. Acta Hydroch Hydrob 27, 38–42 (1999)
Buschmann, H.-J., Cleve, E., Jansen, K., Wego, A., Schollmeyer, E.: Complex formation between cucurbit[n]urils and alkali, alkaline earth and ammonium ions in aqueous solution. J. Incl. Phenom. Macro. Chem. 40, 117–120 (2001)
Wyman, I.W., Macartney, D.H.: Cucurbit[7]uril host-guest complexes with small polar organic guests in aqueous solution. Org. Biomol. Chem. 6, 1796–1801 (2008)
United State Environmental Protection Agency (US EPA), Office of Pesticide Program. www.epa.gov/pesticides/reregistration/thiabendazole. Accessed 25 Jan 2010
Acknowledgments
We acknowledge the support of the National Natural Science Foundation of China (Grant No. 20972034), Natural Science fund of the Science and Technology Department of GuiZhou Province (Grant No. J-2009-2288), Introduced Talents Start-up Project of GuiZhou University (Grant No.2009023), and the Foundation of the Governor of Guizhou Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, Y., Wang, J., Xue, SF. et al. Determination of thiabendazole in aqueous solutions using a cucurbituril-enhanced fluorescence method. J Incl Phenom Macrocycl Chem 72, 397–404 (2012). https://doi.org/10.1007/s10847-011-9999-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-9999-1