Abstract
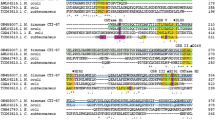
Amylomaltase catalyzes the formation of large-ring cyclodextrins (LR-CDs) from starch. This study aims to construct the recombinant amylomaltase from Corynebacterium glutamicum and to characterize the purified enzyme with the emphasis on the profile of LR-CDs production. A novel amylomaltase from Corynebacterium glutamicum ATCC 13032 was cloned and expressed in Escherichia coli BL21 (DE3) using the expression vector pET-19b. The open reading frame of amylomaltase gene of 2,121 bp (encoding the polypeptide of 706 amino acid residues) was obtained with the N-terminal His-tag fragment of 69 bp attached before the start codon of the amylomaltase gene. The deduced amino acid sequence showed a low sequence identity (20–25%) to those thermostable amylomaltases from Thermus sp. The maximum enzyme activity was obtained when the recombinant cells were cultured at 37 °C for 2 h after induction with 0.4 mM isopropyl thio-β-D-galactoside (IPTG). The enzyme was 11-fold purified with a yield of 30% by a HiTrap affinity column. The purified amylomaltase showed a single band of 84 kDa on a 7.5% SDS-PAGE. When the enzyme acted on pea starch, it catalyzed an intramolecular transglucosylation (cyclization) reaction that produced LR-CDs or cycloamyloses (CA). The product profile was dependent on the incubation time and the enzyme concentration. Shorter incubation time gave larger LR-CDs as principal products. At 4 h incubation, the product was composed of a mixture of LR-CDs in the range of CD19–CD50, with CD27–28 as products with highest amount. It is noted that CD19 was the smallest product in all conditions tested. The enzyme also catalyzes intermolecular transglucosylation on various malto-oligosaccharides, with maltose as the smallest substrate.





Similar content being viewed by others
Abbreviations
- LR-CD:
-
Large-ring cyclodextrins
- CA:
-
Cycloamylose
- CD:
-
Cyclodextrin
- 4αGTase:
-
4-α-Glucanotransferase
- IPTG:
-
Isopropylthio-β-D-galactoside
- SDS-PAGE:
-
Sodium dodecylsulfate-polyacrylamide gel electrophoresis
- TLC:
-
Thin-layer chromatography
- HPAEC:
-
High performance anion exchange chromatography
References
Takaha, T., Smith, S.M.: The functions of 4-α-glucanotransferase and their use for the production of cyclic glucans. Biotechnol. Genet. Eng. Rev. 16, 257–280 (1999)
Gessler, K., Usόn, I., Takaha, T., Krauss, N., Smith, S.M., Okada, S., Sheldrick, G.M., Saenger, W.: V-Amylose at atomic resolution: X-ray structure of a cycloamylose with 26 glucose residues (cyclomaltohexaicosaose). Proc. Natl. Acac. Sci. 96, 4246–4251 (1999)
Kitamura, S., Nakatani, K., Takaha, T., Okada, S.: Complex formation of large-ring cyclodextrins with iodine inaqueous solution as revealed by isothermal titration calorimetry. Macromol. Rapid. Commun. 20, 612–615 (1999)
Endo, T., Zheng, M., Zimmermann, W.: Enzymatic synthesis and analysis of large-ring cyclodextrins. Aust. J. Chem. 55, 39–48 (2002)
Tomono, K., Mugishima, A., Suzuki, T., Goto, H., Ueda, H., Nagai, T., Watanabe, J.: Interaction between cycloamylose and various drugs. J. Incl. Phenom. Macro. 44, 267–270 (2002)
Satake H, Uehori Y, Satou T, Takaba T, Kuriki T, Takada H, Okada S (1998) Coating material for gate roll coater. Japanese Patent, Publication number, 10-219593
Machida, S., Ogawa, S., Xiaohua, S., Takaha, T., Fujii, K., Hayashi, K.: Cycloamylose as an efficient artificial chaperone for protein refolding. FEBS Lett. 486, 131–135 (2000)
Monod, J., Torriani, A.M.: Amylomaltase of Escherichia coli. Ann. Inst. Pasteur (Paris). 78(1), 65–77 (1950)
Goda, S.K., Eissa, O., Akhtar, M., Minton, N.P.: Molecular analysis of a Clostridium butyricum NCIMB 7423 gene encoding 4-alpha-glucanotransferase and characterization of the recombinant enzyme produced in Escherichia coli. Microbiology 143, 3287–3294 (1997)
Jeon, B.S., Taguchi, H., Sakai, H., Ohshima, T., Wakagi, T., Matsuzawa, H.: 4-alpha-glucanotransferase from the hyperthermophilic archaeon Thermococcus litoralis: enzyme purification and characterization, and gene cloning, sequencing and expression in Escherichia coli. Eur. J. Biochem. 248, 171–178 (1997)
Terada, Y., Fujii, K., Takaha, T., Okada, S.: Thermus aquaticus ATCC 33923 amylomaltase gene cloning and expression and enzyme characterization: production of cycloamylose. Appl. Environ. Microbiol. 65, 910–915 (1999)
Bhuiyan, S.H., Kitaoka, M.: Hayashi, K.: A cycloamylose-forming hyperthermostable 4-α-glucanotransferase of Aquifex aeolicus expressed in Escherichia coli. J. Mol. Catal. B Enzym. 22, 45–53 (2003)
Bo-young, B., Kim, H., Kim, H., Baik, M., Ahn, S., Kim, C., Park, C.: Cloning and overexpression of 4-α-glucanotransferase from Thermus brockianus (TBGT) in E. coli. J. Microbiol. Biotechnol. 16, 1809–1813 (2006)
Kalinowski, J., Bathe, B., Bartels, D., Bischoff, N., Bott, M., Burkovski, A., Dusch, N., Eggeling, L., Eikmanns, B.J., Gaigalat, L., Goesmann, A., Hartmann, M., Huthmacher, K., Krämer, R., Linke, B., McHardy, A.C., Meyer, F., Möckel, B., Pfefferle, W., Pühler, A., Rey, D.A., Rückert, C., Rupp, O., Sahm, H., Wendisch, V.F., Wiegräbe, I., Tauch, A.: The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J. Biotechnol. 104, 5–25 (2003)
Ikeda, M., Nakagawa, S.: The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 62, 99–109 (2003)
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl KL (1995) Short protocols in molecular biology, 3rd edn, pp. 2–12. Wiley, USA
Park, J., Kim, H., Kim, Y., Cha, H., Kim, Y., Kim, T., Kim, Y., Park, K.: The action mode of Thermus aquaticus YT-1 4-α-glucanotransferase and its chimeric enzymes introduced with starch-binding domain on amylose and amylopectin. Carbohydr. Polym. 67, 164–173 (2007)
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970)
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72, 248–254 (1976)
Takaha, T., Yanase, M., Okada, S., Smith, S.M.: Disproportionating Enzyme (4-α-Glucanotransferase; EC 2.4.1.25) of Potato. Purification, Molecular cloning, and potential role in starch metabolism. J. Biol. Chem. 268, 1391–1396 (1993)
Kakefuda, G., Duke, S.H.: Characterization of pea-chloroplast D-enzyme. Plant Physiol. 91, 136–143 (1989)
Takaha, T., Yanase, M., Takata, H., Okada, S., Smith, S.M.: Potato D-enzyme catalyzes the cyclization of amylose to produce cycloamylose, a novel cyclic glucan. J. Biol. Chem. 271(6), 2902–2908 (1996)
Acknowledgments
WS was financially supported by the Royal Golden Jubilee PhD Fellowship from the Thailand Research Fund. Financial support from the Ratchadapiseksomphot Endowment Fund of Chulalongkorn University to the Starch and Cyclodextrin Research Unit and from Research Institution Partnership Grant of Alexander von Humboldt Foundation are acknowledged. The authors also acknowledge the support from the Thai Government Stimulus Package 2 (TKK 2555) under the Project PERFECTA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srisimarat, W., Powviriyakul, A., Kaulpiboon, J. et al. A novel amylomaltase from Corynebacterium glutamicum and analysis of the large-ring cyclodextrin products. J Incl Phenom Macrocycl Chem 70, 369–375 (2011). https://doi.org/10.1007/s10847-010-9890-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9890-5