Abstract
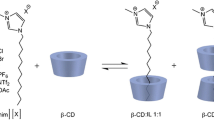
The inclusion complexes between fusidate, 3-keto fusidate, 11-keto fusidate and 11-deoxy fusidate and α-, β-, and γ-cyclodextrin (CD) were studied using capillary electrophoresis. By monitoring the changes in mobility of the negatively charged compounds in the presence of varying amount of CD the stability constants of the complexes formed could be obtained. In the case of α- and β-CD the obtained results could be modelled to a simple model assuming 1:1 stoichiometry, revealing, not surprisingly, that β-CD formed a stronger complex compared to α-CD. A model assuming 1:2 (fusidate:CD) stoichiometry could be fitted to the data obtained with γ-CD. The results showed that the different fusidanes formed very strong 1:1 complexes with γ-CD as well as a quite weak 1:2 complex. 3-keto-, 11-keto- and 11-deoxy-fusidate formed stronger complexes compared to fusidate, probably due to an decrease in hydrophilicity caused by the reduced number of hydroxyl groups. The complex between γ-CD and fusidate was studied by use of 2D-NMR spectroscopy. The results showed that most of the hydrogen atoms of fusidate show interactions with the hydrogen atoms in the cavity of γ-CD. The interaction pattern suggests that fusidate may be fully embedded in the cavity of γ-CD. No interactions between fusidate and the hydrogen atoms situated at the outside of the CD were found.





Similar content being viewed by others
Abbreviations
- CD:
-
Cyclodextrin
- COSY:
-
Correlation spectroscopy
- DQF:
-
Double Quantum Filtered
- ROESY:
-
Rotating Overhauser Effect Spectroscopy
References
Wilkinson, J.D.: Fusidic acid in dermatology. Br. J. Dermatol. Suppl. 139, 37–40 (1998)
Turnidge, J.: Fusidic acid pharmacology, pharmacokinetics and pharmacodynamics. Int. J. Antimicrob. Agents. 12, S23 (1999)
Habon, I., Stadler-Szöke, A., Szejtli, J.: Improvement of the solubility of steroids by formation of cyclodextrin inclusion complex. Acta. Biochim. Biophys. Acad. Sci. Hung. 19, 86 (1984)
Ahmed, S.M.: Improvement of solubility and dissolution of 19-norprogesterone via inclusion complexation. J. Inclusion Phenom. 30, 111–125 (1998)
Uekama, K., Otagiri, M., Uemura, Y., Fujinaga, T., Arimori, K., Matsuo, N., Tasaki, K., Sugii, A.: Improvement of oral bioavailability of prednisolone by beta-cyclodextrin complexation in humans. J. Pharmacobiodyn 6, 124–127 (1983)
Uekama, K., Sakai, A., Arimori, K., Otagiri, M., Saitô, H.: Different mode of prednisolone within alpha-cyclodextrins, beta-cyclodextrins and gamma-cyclodextrins in aqueous-solution and in solid state. Pharm. Acta. Helv. 60, 117–121 (1985)
Uekama, K., Fujinaga, T., Otagiri, M., Yamasaki, M.: Inclusion complexations of steroid-hormones with cyclodextrins in water and solid-phase. Int. J. Pharm. 10, 1–15 (1982)
Liu, F.Y., Kildsig, D.O., Mitra, A.K.: Beta-cyclodextrin steroid complexation – Effects of steroid structure on association equilibria. Pharm. Res. 7, 869–873 (1990)
Djedaïni, F., Perly, B.: Nuclear-Magnetic-Resonance investigation of the stoichiometries in beta-cyclodextrin-steroid inclusion complexes. J. Pharm. Sci. 80, 1157–1161 (1991)
Marzona, M., Carpignano, R., Quargliotto, P.: Quantitative structure-stability relationships in the inclusion complexes of steroids with cyclodextrins. Ann. Chim. 82, 517–537 (1992)
Forgo, P., Vincze, I., Kover, K.E.: Inclusion complexes of ketosteroids with beta-cyclodextrin. Steroids 68, 321–327 (2003)
Cabrer, P.R., Alvarez-Parrilla, E., Meijide, F., Seijas, J.A., Nunez, E.R., Tato, J.V.: Complexation of sodium cholate and sodium deoxycholate by beta-cyclodextrin and derivatives. Langmuir 15, 5489–5495 (1999)
Pean, C., Creminon, C., Wijkhuisen, A., Perly, B., Djedaïni-Pilard, F.: Reliable NMR experiments for the study of beta-cyclodextrin/prostaglandin E-2 inclusion complex. J. Chim. Phys. Phys. Chim. Biol. 96, 1486–1493 (1999)
Forgo, P., Göndös, G.: A study of beta-cyclodextrin inclusion complexes with progesterone and hydrocortisone using rotating frame Overhauser spectroscopy. Monatsh. Chem. 133, 101–106 (2002)
Cameron, K.S., Fletcher, D., Fielding, L.: An NMR study of cyclodextrin complexes of the steroidal neuromuscular blocker drug Rocuronium Bromide. Magn. Reson. Chem. 40, 251–260 (2002)
Bednarek, E., Bocian, W., Poznanski, J., Sitkowski, J., Sadlej-Sosnowska, N., Kozerski, L.: Complexation of steroid hormones: prednisolone, ethinyloestradiol and estriol with beta-cyclodextrin. An aqueous H-1 NMR study. J. Chem. Soc. Perkin. Trans. 2, 999–1004 (2002)
Jover, A., Budal, R.M., Al-Soufi, W., Meijide, F., Tato, J.V., Yunes, R.A.: Spectra and structure of complexes formed by sodium fusidate and potassium helvolate with beta- and gamma-cyclodextrin. Steroids 68, 55–64 (2003)
Al-Soufi, W., Cabrer, P.R., Jover, A., Budal, R.M., Tato, J.V.: Determination of second-order association constants by global analysis of H-1 and C-13 NMR chemical shifts. Application to the complexation of sodium fusidate and potassium helvolate by beta- and gamma-cyclodextrin. Steroids 68, 43–53 (2003)
Larsen, K.L., Aachmann, F.L., Wimmer, R., Stella, V.J., Madsen Kjølner, U.: Phase solubility and structure of the inclusion complexes of prednisolone and 6 alpha-methyl prednisolone with various cyclodextrins. J. Pharm. Sci. 94, 507–515 (2005)
Bartels, C., Xia, T.-H., Billeter, M., Günter, P., Wüthrich, K.: The program XEASY for computer-supported NMR spectral-analysis of biological macromolecules. J. Biomol. NMR 5, 1–10 (1995)
Larsen, K.L., Zimmerman, W.: Analysis and characterisation of cyclodextrins and their complexes by affinity capillary electrophoresis. J. Chromatogr. A. 836, 3–14 (1999)
Lee, Y., Lin, I.: Capillary electrophoretic analysis of cyclodextrins and determination of formation constants for inclusion complexes. Electrophoresis 17, 333–340 (1996)
Larsen, K.L., Endo, T., Ueda, H., Zimmerman, W.: Inclusion complex formation constants of alpha-, beta-, gamma-, delta-, epsilon-, zeta-, eta-, and theta-cyclodextrins determined with capillary zone electrophoresis. Carbohydr. Res. 309, 153–159 (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larsen, K.L., Andersen, S.B., Mørkbak, A.L. et al. Inclusion complexes of fusidic acid and three structurally related compounds with cyclodextrins. J Incl Phenom Macrocycl Chem 57, 185–190 (2007). https://doi.org/10.1007/s10847-006-9198-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-006-9198-7