Abstract
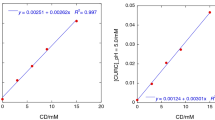
The aim of this work is to increase the stability and water solubility of resveratrol by complexation with different cyclodextrins. Furthermore, physical–chemical properties of each inclusion compound were investigated. Complexes of resveratrol with cyclodextrins both native (α, β, γ) and modified (2-hydroxypropyl-β-cyclodextrin, dimethyl-β-cyclodextrin) were obtained by using the suspension method. An inclusion complex with β-cyclodextrin was also prepared by using the microwave. Solid state characterization of the products was carried out using Fourier transform infrared spectroscopy (FT-IR), differential scanning calorimetry (DSC), X-ray diffraction (DRX); solution studies were performed by UV–Vis spectrophotometry and 1H-NMR spectroscopy. Phase solubility profiles with all cyclodextrins employed were classified as AN type, indicating the formation of 1:1 stoichiometric inclusion complexes. Stability constants (K c) from the phase solubility diagrams were calculated. Stability studies in the solid state and in solution were performed; the photodegradation by UV–Vis spectrophotometry was monitored. The isomerization rate trans to cis, in ethanol solution, decreased with inclusion. The dissolution studies revealed that resveratrol dissolution rate was improved by the formation of inclusion complexes.
Similar content being viewed by others
References
Lamuela-Raventos R.M., Romero-Perez A.I., Waterhouse A.L., de la Torre-Boronat M.C.: (1995) J. Agric. Food Chem. 43: 281
Romero-Perez A.I., Ibern-Gomez M., Lamuela-Raventos R.M., de la Torre-Boronat M.C.: (1999). J. Agric. Food Chem. 47: 1533
Dixon R.A.: (2001) Nature 411: 843
Nonomura S., Kanagawa H., Makimoto A.: (1963). Yakugaku Zasshi 83: 988
Renaud S. de, Lorgeril M. (1992). Lancet 339: 1523
Constant J.: (1997) Coron. Artery Dis. 8: 645
Kimura Y., Okuda H., Arichi S.: (1985) Biochim. Biophys. Acta 837: 209
Bertelli A.A.E, Giovannini L., Giannessi D., Migliori M., Bernini W., Fregani M., Bertelli A.: (1995) Int. J. Tissue React. 17: 1
Frankel E.N., Waterhouse A.L., Kinsella J.E.: (1993). Lancet 341: 1103
Belguendouz L., Fremont L., Gozzelino M.T.: (1998). Biochem. Pharmacol. 55: 811
Chanvitayapongs S., Draczynska-Lusiak B., Sun A.Y.: (1997). Neuroreport 8: 1499
Jang M., Cai L., Udeani G., Slowing K.V., Thomas C.F., Pezzuto J.M.: (1997) Science 275: 218
Clement M.V., Hirpara J.L., Chawdhuryet S., Pervaiz S.: (1998) Blood 92: 996
Fontecave M., Lepoivre M., Elleingand E., Gerez C., Guittet O.: (1998) FEBS Lett. 421: 277
Chan M.M.Y.: (2002). Biochem. Pharm. 63: 99
Rajewski R.A., Stella V.J.: (1996). J. Pharm. Sci. 85: 1142
Duchêne D., Wouessidjewe D., Ponchel G.: (1999) J. Control. Release 62: 263
Fromming K., Szejtli J.: (1994) Cyclodextrins in Pharmacy, Kluwer Acad. Publ., Dordrecht
Wang Y., Catana F., Yang Y., Roderick R., van Breemen R.B.: (2002) J. Agr. Food Chem. 50: 431
Mattivi F.: (1993) Z. Lebensm. Unters. Forsch. 196: 522
Wen X., Tan F., Jing Z., Liu Z.: (2004) J. Pharm. Biom. Anal. 34: 517
Higuchi T., Connors K.A.: (1965) Adv. Anal. Chem. Instr. 4: 117
ICH, Harmonized Tripartite Guideline (Q1B), November 1996
Mura P., Maestrelli F., Cirri M., Furlanetto S., Pinzauti S.: (2003). J. Thermal Anal. Cal. 74:635
Dollo G., Le Corre P., Chevanne F., Le Verge R.: (1996). Int. J. Pharm. 131: 219
Nozawa Y., Morioka Y., Sadzuka Y., Miyagishima A., Hirota S., Guillory J.K.: (1997). Pharm. Acta Helv. 72: 113
Astakova A.V., Demin N.B.: (2004). Pharma. Chem. J. 38: 46
Uekama K., Fujise A., Otagiri M., Hirayama F., Inaba K.: (1984) Chem. Pharm. Bull. 32: 275
Calabrò M.L., Tommasini S., Donato P., Ranieri D., Stancanelli R., Ficarra P., Ficarra R., Costa C., Catania S., Rustichelli C., Gamberini G.: (2004). J. Pharm. Biom. Anal. 35: 364
Trapani G., Latrofa A., Franco M., Lopedota A., Sanna E., Liso G.: (1998). J. Pharm. Sci. 87: 514
Ueda H., Nagai T.: (1980). Chem. Pharm. Bull. 28: 1415
Cabral−Marques H.M., Hadgraft J., Kellaway I.W., Pugh W.J.: (1990). Int. J. Pharm. 63: 267
Fronza G., Mele A., Redenti E., Ventura P.: (1992). J. Pharm. Sci. 81: 1162
Moyano J.R., Arias−Bianco M.J., Gines J.M., Rabasco A.M., Perez−Martinez J.I., Mor M., Giordano M.: (1997) J. Pharm. Sci. 86: 72
Djedani F., Perly B.: (1991), NMR of cyclodextrins, derivatives and inclusion compounds. In: Duchêne D., (Ed.), New Trends in Cyclodextrins and Derivatives, Editions de Santé , Paris pp.217–246
Harata K.: (1990), Macrocyclic conformation of methylated cyclodestrins. In: Duchêne D., (Ed.), Minutes 5th International Symposium on Cyclodextrins, Editions de Santé , Paris pp.77–81
Smolkova-Keulemansova E., Feltl L., Snopek J, (1990). Cyclodextrins and their derivatives in modern analytical high-performance separation methods. In: Duchêne D., (Ed.), Minutes 5th International Symposium on Cyclodextrins, Editions de Santé , Paris, pp.617–622
Acknowledgements
We are indebted to our Professor Riccardo Stradi (Istituto di Chimica Organica “A.Marchesini”, Università degli Studi di Milano, Facoltà di Farmacia) for the helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertacche, V., Lorenzi, N., Nava, D. et al. Host–Guest Interaction Study of Resveratrol With Natural and Modified Cyclodextrins. J Incl Phenom Macrocycl Chem 55, 279–287 (2006). https://doi.org/10.1007/s10847-006-9047-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-006-9047-8