Abstract
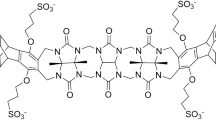
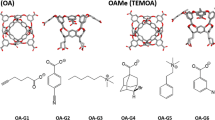
In this effort in the SAMPL6 host–guest binding challenge, a combination of molecular dynamics and quantum mechanical methods were used to blindly predict the host–guest binding free energies of a series of cucurbit[8]uril (CB8), octa-acid (OA), and tetramethyl octa-acid (TEMOA) hosts bound to various guest molecules in aqueous solution. Poses for host–guest systems were generated via molecular dynamics (MD) simulations and clustering analyses. The binding free energies for the structures obtained via cluster analyses of MD trajectories were calculated using the MMPBSA method and density functional theory (DFT) with the inclusion of Grimme’s dispersion correction, an implicit solvation model to model the aqueous solution, and the resolution-of-the-identity (RI) approximation (MMPBSA, RI-B3PW91-D3, and RI-B3PW91, respectively). Among these three methods tested, the results for OA and TEMOA systems showed MMPBSA and RI-B3PW91-D3 methods can be used to qualitatively rank binding energies of small molecules with an overbinding by 7 and 37 kcal/mol respectively, and RI-B3PW91 gave the poorest quality results, indicating the importance of dispersion correction for the binding free energy calculations. Due to the complexity of the CB8 systems, all of the methods tested show poor correlation with the experimental results. Other quantum mechanical approaches used for the calculation of binding free energies included DFT without the RI approximation, utilizing truncated basis sets to reduce the computational cost (memory, disk space, CPU time), and a corrected dielectric constant to account for ionic strength within the implicit solvation model.








Similar content being viewed by others
References
Klepeis JL, Lindorff-Larsen K, Dror RO, Shaw DE (2009) Long-timescale molecular dynamics simulations of protein structure and function. Curr Opin Struct Biol 19:120–127. https://doi.org/10.1016/j.sbi.2009.03.004
Shan Y, Seeliger MA, Eastwood MP et al (2009) A conserved protonation-dependent switch controls drug binding in the Abl kinase. Proc Natl Acad Sci USA 106:139–144. https://doi.org/10.1073/pnas.0811223106
Zhao G, Perilla JR, Yufenyuy EL et al (2013) Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 497:643–646. https://doi.org/10.1038/nature12162
Perilla JR, Goh BC, Cassidy CK et al (2015) Molecular dynamics simulations of large macromolecular complexes. Curr Opin Struct Biol 31:64–74. https://doi.org/10.1016/j.sbi.2015.03.007
Walkowicz WE, Fernández-Tejada A, George C et al (2016) Quillaja saponin variants with central glycosidic linkage modifications exhibit distinct conformations and adjuvant activities. Chem Sci 7:2371–2380. https://doi.org/10.1039/C5SC02978C
Hadden JA, Perilla JR, Schlicksup CJ et al (2018) All-atom molecular dynamics of the HBV capsid reveals insights into biological function and cryo-EM resolution limits. Elife 7:e32478. https://doi.org/10.7554/eLife.32478
García MA, Meurs EF, Esteban M (2007) The dsRNA protein kinase PKR: virus and cell control. Biochimie 89:799–811. https://doi.org/10.1016/j.biochi.2007.03.001
Tripathi RB, Pande M, Garg G, Sharma D (2016) In-silico expectations of pharmaceutical industry to design of new drug molecules. J Innov Pharm Biol Sci 3:95–103
Ryde U, Söderhjelm P (2016) Ligand-binding affinity estimates supported by quantum-mechanical methods. Chem Rev 116:5520–5566. https://doi.org/10.1021/acs.chemrev.5b00630
Ganesan A, Coote ML, Barakat K (2017) Molecular dynamics-driven drug discovery: leaping forward with confidence. Drug Discov Today 22:249–269. https://doi.org/10.1016/j.drudis.2016.11.001
Mobley DL, Gilson MK (2017) Predicting binding free energies: frontiers and benchmarks. Annu Rev Biophys 46:531–558. https://doi.org/10.1146/annurev-biophys-070816-033654
Huggins DJ, Sherman W, Tidor B (2012) Rational approaches to improving selectivity in drug design. J Med Chem 55:1424–1444. https://doi.org/10.1021/jm2010332
Muddana HS, Varnado CD, Bielawski CW et al (2012) Blind prediction of host–guest binding affinities: a new SAMPL3 challenge. J Comput Aided Mol Des 26:475–487. https://doi.org/10.1007/s10822-012-9554-1
Rogers KE, Ortiz-Sánchez JM, Baron R et al (2013) On the role of dewetting transitions in host-guest binding free energy calculations. J Chem Theory Comput 9:46–53. https://doi.org/10.1021/ct300515n
Yang H, Yuan B, Zhang X, Scherman OA (2014) Supramolecular chemistry at interfaces: host-guest interactions for fabricating multifunctional biointerfaces. Acc Chem Res 47:2106–2115. https://doi.org/10.1021/ar500105t
Muddana HS, Fenley AT, Mobley DL, Gilson MK (2014) The SAMPL4 host–guest blind prediction challenge: an overview. J Comput Aided Mol Des 28:305–317. https://doi.org/10.1007/s10822-014-9735-1
Gallicchio E, Chen H, Chen H et al (2015) BEDAM binding free energy predictions for the SAMPL4 octa-acid host challenge. J Comput Aided Mol Des 29:315–325. https://doi.org/10.1007/s10822-014-9795-2
Yin J, Henriksen NM, Slochower DR et al (2017) Overview of the SAMPL5 host–guest challenge: are we doing better? J Comput Aided Mol Des 31:1–19. https://doi.org/10.1007/s10822-016-9974-4
Liu S, Ruspic C, Mukhopadhyay P et al (2005) The cucurbit[n]uril family: prime components for self-sorting systems. J Am Chem Soc 127:15959–15967. https://doi.org/10.1021/ja055013x
Gan H, Benjamin CJ, Gibb BC (2011) Nonmonotonic assembly of a deep-cavity cavitand. J Am Chem Soc 133:4770–4773. https://doi.org/10.1021/ja200633d
Biedermann F, Scherman OA (2012) Cucurbit[8]uril mediated donor–acceptor ternary complexes: a model system for studying charge-transfer interactions. J Phys Chem B 116:2842–2849. https://doi.org/10.1021/jp2110067
Vázquez J, Remón P, Dsouza RN et al (2014) A simple assay for quality binders to cucurbiturils. Chemistry 20:9897–9901. https://doi.org/10.1002/chem.201403405
Gibb CLD, Gibb BC (2014) Binding of cyclic carboxylates to octa-acid deep-cavity cavitand. J Comput Aided Mol Des 28:319–325. https://doi.org/10.1007/s10822-013-9690-2
Nicholls A, Wlodek S, Grant JA (2009) The SAMP1 solvation challenge: further lessons regarding the pitfalls of parametrization. J Phys Chem B 113:4521–4532. https://doi.org/10.1021/jp806855q
Mobley DL, Bayly CI, Cooper MD, Dill KA (2009) Predictions of hydration free energies from all-atom molecular dynamics simulations. J Phys Chem B 113:4533–4537. https://doi.org/10.1021/jp806838b
Geballe MT, Skillman AG, Nicholls A et al (2010) The SAMPL2 blind prediction challenge: introduction and overview. J Comput Aided Mol Des 24:259–279. https://doi.org/10.1007/s10822-010-9350-8
Zwanzig RW (1954) High-temperature equation of state by a perturbation method. I. Nonpolar gases. J Chem Phys 22:1420–1426. https://doi.org/10.1063/1.1740409
Jiang W, Hodoscek M, Roux B (2009) Computation of absolute hydration and binding free energy with free energy perturbation distributed replica-exchange molecular dynamics. J Chem Theory Comput 5:2583–2588. https://doi.org/10.1021/ct900223z
Mitchell MJ, McCammon JA (1991) Free energy difference calculations by thermodynamic integration: difficulties in obtaining a precise value. J Comput Chem 12:271–275. https://doi.org/10.1002/jcc.540120218
Chodera JD, Mobley DL, Shirts MR et al (2011) Alchemical free energy methods for drug discovery: progress and challenges. Curr Opin Struct Biol 21:150–160. https://doi.org/10.1016/j.sbi.2011.01.011
Hansen N, van Gunsteren WF (2014) Practical aspects of free-energy calculations: a review. J Chem Theory Comput 10:2632–2647. https://doi.org/10.1021/ct500161f
Williams-Noonan BJ, Yuriev E, Chalmers DK (2018) Free energy methods in drug design: prospects of “Alchemical Perturbation” in medicinal chemistry. J Med Chem 61:638–649. https://doi.org/10.1021/acs.jmedchem.7b00681
Hou T, Wang J, Li Y, Wang W (2011) Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J Chem Inf Model 51:69–82. https://doi.org/10.1021/ci100275a
Homeyer N, Gohlke H (2012) Free energy calculations by the molecular mechanics Poisson–Boltzmann surface area method. Mol Inform 31:114–122. https://doi.org/10.1002/minf.201100135
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10:449–461. https://doi.org/10.1517/17460441.2015.1032936
Wang C, Greene D, Xiao L et al (2018) Recent developments and applications of the MMPBSA method. Front Mol Biosci. https://doi.org/10.3389/fmolb.2017.00087
Genheden S, Ryde U (2011) Comparison of the efficiency of the LIE and MM/GBSA methods to calculate ligand-binding energies. J Chem Theory Comput 7:3768–3778. https://doi.org/10.1021/ct200163c
Hansson T, Marelius J, Aqvist J (1998) Ligand binding affinity prediction by linear interaction energy methods. J Comput Aided Mol Des 12:27–35. https://doi.org/10.1023/A:1007930623000
Steinmann C, Olsson MA, Ryde U (2018) Relative ligand-binding free energies calculated from multiple short QM/MM MD simulations. J Chem Theory Comput. https://doi.org/10.1021/acs.jctc.8b00081 Article ASAP
Curutchet C, Cupellini L, Kongsted J et al (2018) Density-dependent formulation of dispersion-repulsion interactions in hybrid multiscale quantum/molecular mechanics (QM/MM) models. J Chem Theory Comput 14:1671–1681. https://doi.org/10.1021/acs.jctc.7b00912
Sellers BD, James NC, Gobbi A (2017) A comparison of quantum and molecular mechanical methods to estimate strain energy in drug like fragments. J Chem Inf Model 57:1265–1275. https://doi.org/10.1021/acs.jcim.6b00614
Lu Y, Yang CY, Wang S (2006) Binding free energy contributions of interfacial waters in HIV-1 protease/inhibitor complexes. J Am Chem Soc 128:11830–11839. https://doi.org/10.1021/ja058042g
Bonnet P, Bryce RA (2004) Molecular dynamics and free energy analysis of neuraminidase–ligand interactions. Protein Sci 13:946–957. https://doi.org/10.1110/ps.03129704.four-hydroxyl
Kitamura K, Tamura Y, Ueki T et al (2014) Binding free-energy calculation is a powerful tool for drug optimization: calculation and measurement of binding free energy for 7-azaindole derivatives to glycogen synthase kinase-3β. J Chem Inf Model 54:1653–1660. https://doi.org/10.1021/ci400719v
Caldararu O, Olsson MA, Riplinger C et al (2017) Binding free energies in the SAMPL5 octa-acid host–guest challenge calculated with DFT-D3 and CCSD(T). J Comput Aided Mol Des 31:87–106. https://doi.org/10.1007/s10822-016-9957-5
Sure R, Antony J, Grimme S (2014) Blind prediction of binding affinities for charged supramolecular host-guest systems: achievements and shortcomings of DFT-D3. J Phys Chem B 118:3431–3440. https://doi.org/10.1021/jp411616b
Mikulskis P, Cioloboc D, Andrejić M et al (2014) Free-energy perturbation and quantum mechanical study of SAMPL4 octa-acid host-guest binding energies. J Comput Aided Mol Des 28:375–400. https://doi.org/10.1007/s10822-014-9739-x
Murkli S, McNeil J, Isaacs L (2018) CB[8]-guest binding affinities: a blinded dataset for the SAMPL6 challenge. Supramol Chem (Submitted)
Neese F, Wennmohs F, Hansen A, Becker U (2009) Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem Phys 356:98–109. https://doi.org/10.1016/j.chemphys.2008.10.036
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Mintz B, Lennox KP, Wilson AK (2004) Truncation of the correlation consistent basis sets: an effective approach to the reduction of computational cost? J Chem Phys 121:5629–5634. https://doi.org/10.1063/1.1785145
Molecular Operating Environment (MOE) (2016) Chemical Computing Group Inc., Montreal
Corbeil CR, Williams CI, Labute P (2012) Variability in docking success rates due to dataset preparation. J Comput Aided Mol Des 26:775–786. https://doi.org/10.1007/s10822-012-9570-1
Hoffmann R (1963) An extended Hückel Theory. I. Hydrocarbons. J Chem Phys 39:1397–1412. https://doi.org/10.1063/1.1734456
Hornak V, Abel R, Okur A et al (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65:712–725. https://doi.org/10.1002/prot.21123
Wang J, Wolf RM, Caldwell JW et al (2004) Development and testing of a general Amber force field. J Comput Chem 25:1157–1174. https://doi.org/10.1002/jcc.20035
Jakalian A, Jack DB, Bayly CI (2002) Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J Comput Chem 23:1623–1641. https://doi.org/10.1002/jcc.10128
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222. https://doi.org/10.1007/BF00533485
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283. https://doi.org/10.1063/1.448799
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 Revision A.03. Gaussian Inc., Wallingford CT.
Case DA, Betz RM, Cerutti DS, Cheatham TE III, Darden TA, Duke RE, Giese TJ, Gohlke H, Goetz AW, Homeyer N, Izadin S, Janowski P, Kaus J, Kovalenko A, Lee TS, LeGrand S, Li P, Lin C, Luchko T, Luo R, Madej B, Mermelstein D, Merz KM, Monard G, Nguyen H, Nguyen HT, Omelyan I, Onufriev A, Roe DR, Roitberg A, Sagui C, Simmerling CL, Botello-Smith WM, Swails J, Walker RC, Wang J, Wolf RM, Wu X, Xiao L and Kollman PA (2016) AMBER 2016. University of California, San Francisco
Joung IS, Cheatham TE (2008) Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J Phys Chem B 112:9020–9041. https://doi.org/10.1021/jp8001614
Horn HW, Swope WC, Pitera JW et al (2004) Development of an improved four-site water model for biomolecular simulations: TIP4P-Ew. J Chem Phys 120:9665–9678. https://doi.org/10.1063/1.1683075
Miller BR, McGee TD, Swails JM et al (2012) MMPBSA.py: an efficient program for end-state free energy calculations. J Chem Theory Comput 8:3314–3321. https://doi.org/10.1021/ct300418h
Ester M, Kriegel H-P, Sander J, Xu X (1996) A density-based algorithm for discovering clusters in large spatial databases with noise. In: KDD-96 proceedings, pp 226–231
Merrick JP, Moran D, Radom L (2007) An evaluation of harmonic vibrational frequency scale factors. J Phys Chem A 111:11683–11700. https://doi.org/10.1021/jp073974n
Neese F (2018) Software update: the ORCA program system, version 4.0. Wiley Interdiscip Rev Comput Mol Sci 8:e1327. https://doi.org/10.1002/wcms.1327
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45:13244–13249. https://doi.org/10.1103/PhysRevB.45.13244
Perdew JP, Chevary JA, Vosko SH et al (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671–6687. https://doi.org/10.1103/PhysRevB.46.6671
Eichkorn K, Treutler O, Öhm H et al (1995) Auxiliary basis sets to approximate Coulomb potentials. Chem Phys Lett 240:283–290. https://doi.org/10.1016/0009-2614(95)00621-A
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. https://doi.org/10.1021/jp810292n
Goerigk L, Grimme S (2010) A general database for main group thermochemistry, kinetics, and noncovalent interactions—assessment of common and reparameterized (meta-)GGA density functionals. J Chem Theory Comput. https://doi.org/10.1021/ct900489g
Goerigk L, Grimme S (2011) A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys Chem Chem Phys 13:6670. https://doi.org/10.1039/c0cp02984j
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007. https://doi.org/10.1063/1.456153
Feller D (1992) Application of systematic sequences of wave functions to the water dimer. J Chem Phys 96:6104–6114. https://doi.org/10.1063/1.462652
Martin JML (1996) Ab initio total atomization energies of small molecules—towards the basis set limit. Chem Phys Lett 259:669–678. https://doi.org/10.1016/0009-2614(96)00898-6
Wilson AK, Dunning TH Jr (1997) Benchmark calculations with correlated molecular wave functions. X. Comparison with “exact” MP2 calculations on Ne, HF, H2O, and N2. J Chem Phys 106:8718–8726. https://doi.org/10.1063/1.473932
Feller D, Peterson KA, Crawford TD (2006) Sources of error in electronic structure calculations on small chemical systems. J Chem Phys 124:054107. https://doi.org/10.1063/1.2137323
Jensen F (2002) Polarization consistent basis sets. II. Estimating the Kohn–Sham basis set limit. J Chem Phys 116:7372–7379. https://doi.org/10.1063/1.1465405
Faver JC, Zheng Z, Merz KM (2011) Model for the fast estimation of basis set superposition error in biomolecular systems. J Chem Phys 135:144110. https://doi.org/10.1063/1.3641894
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Gavish N, Promislow K (2016) Dependence of the dielectric constant of electrolyte solutions on ionic concentration: a microfield approach. Phys Rev E 94:012611. https://doi.org/10.1103/PhysRevE.94.012611
Kendall MG (1938) A new measure of rank correlation. Biometrika 30:81. https://doi.org/10.2307/2332226
Berry KJ, Johnston JE, Zahran S, Mielke PW (2009) Stuart’s tan measure of effect size for ordinal variables: some methodological considerations. Behav Res Methods 41:1144–1148. https://doi.org/10.3758/BRM.41.4.1144
Dean RB, Dixon WJ (1951) Simplified statistics for small numbers of observations. Anal Chem 23:636–638. https://doi.org/10.1021/ac60052a025
Bayly CI, Cieplak P, Cornell WD, Kollman PA (1993) A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Phys Chem 97:10269–10280. https://doi.org/10.1021/j100142a004
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eken, Y., Patel, P., Díaz, T. et al. SAMPL6 host–guest challenge: binding free energies via a multistep approach. J Comput Aided Mol Des 32, 1097–1115 (2018). https://doi.org/10.1007/s10822-018-0159-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-018-0159-1