Abstract
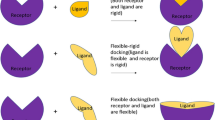
The understanding and optimization of protein-ligand interactions are instrumental to medicinal chemists investigating potential drug candidates. Over the past couple of decades, many powerful standalone tools for computer-aided drug discovery have been developed in academia providing insight into protein-ligand interactions. As programs are developed by various research groups, a consistent user-friendly graphical working environment combining computational techniques such as docking, scoring, molecular dynamics simulations, and free energy calculations is needed. Utilizing PyMOL we have developed such a graphical user interface incorporating individual academic packages designed for protein preparation (AMBER package and Reduce), molecular mechanics applications (AMBER package), and docking and scoring (AutoDock Vina and SLIDE). In addition to amassing several computational tools under one interface, the computational platform also provides a user-friendly combination of different programs. For example, utilizing a molecular dynamics (MD) simulation performed with AMBER as input for ensemble docking with AutoDock Vina. The overarching goal of this work was to provide a computational platform that facilitates medicinal chemists, many who are not experts in computational methodologies, to utilize several common computational techniques germane to drug discovery. Furthermore, our software is open source and is aimed to initiate collaborative efforts among computational researchers to combine other open source computational methods under a single, easily understandable graphical user interface.





Similar content being viewed by others
References
Alvarez JC (2004) High-throughput docking as a source of novel drug leads. Curr Opin Chem Biol 8:365–370
Green DV (2003) Virtual screening of virtual libraries. Prog Med Chem 41:61–97
Ooms F (2000) Molecular modeling and computer aided drug design. Examples of their applications in medicinal chemistry. Curr Med Chem 7:141–158
van de Waterbeemd H (2005) From in vivo to in vitro/in silico ADME: progress and challenges. Expert Opin Drug Metab Toxicol 1:1–4
Trott O, Olson AJ (2010) Software news and update AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Case DA, Cheatham TE, Darden T, Gohlke H, Luo R, Merz KM, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) The Amber biomolecular simulation programs. J Comput Chem 26:1668–1688
Danielson ML, Lill MA (2010) New computational method for prediction of interacting protein loop regions. Proteins 78:1748–1759
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: Algorithms for highly efficient, load-balanced and scalable molecular simulation. J Chem Theory Comput 4:435–447
Pearlman DA, Case DA, Caldwell JW, Ross WS, Cheatham TE, Debolt S, Ferguson D, Seibel G, Kollman P (1995) Amber, a package of computer-programs for applying molecular mechanics, normal-mode analysis molecular-dynamics and free-energy calculations to simulate the structural and energetic properties of molecules. Comput Phys Commun 91:1–41
Seeliger D, de Groot BL (2010) Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des 24:417–422
Soto CS, Fasnacht M, Zhu J, Forrest L, Honig B (2008) Loop modeling: sampling, filtering, and scoring. Proteins 70:834–843
Zavodszky MI, Sanschagrin PC, Korde RS, Kuhn LA (2002) Distilling the essential features of a protein surface for improving protein-ligand docking, scoring, and virtual screening. J Comput Aided Mol Des 16:883–902
de Molfetta FA, de Freitas RF, da Silva AB, Montanari CA (2009) Docking and molecular dynamics simulation of quinone compounds with trypanocidal activity. J Mol Model 15:1175–1184
Graves AP, Shivakumar DM, Boyce SE, Jacobson MP, Case DA, Shoichet BK (2008) Rescoring docking hit lists for model cavity sites: predictions and experimental testing. J Mol Biol 377:914–934
Manetti F, Locatelli GA, Maga G, Schenone S, Modugno M, Forli S, Corelli F, Botta M (2006) A combination of docking/dynamics simulations and pharmacophoric modeling to discover new dual c-Src/Abl kinase inhibitors. J Med Chem 49:3278–3286
Okimoto N, Futatsugi N, Fuji H, Suenaga A, Morimoto G, Yanai R, Ohno Y, Narumi T, Taiji M (2009) High-performance drug discovery: computational screening by combining docking and molecular dynamics simulations. PLoS Comput Biol 5:e1000528
Paulsen JL, Anderson AC (2009) Scoring ensembles of docked protein:ligand interactions for virtual lead optimization. J Chem Inf Model 49:2813–2819
Park IH, Li C (2010) Dynamic ligand-induced-fit simulation via enhanced conformational samplings and ensemble dockings: a survivin example. J. Phys. Chem. B 114:5144–5153
Hritz J, de Ruiter A, Ostenbrink C (2008) Impact of plasticity and flexibility on docking results for cytochrome P450 2D6: a combined approach of molecular dynamics and ligand docking. J Med Chem 51:7469–7477
Berman H, Henrick K, Nakamura H, Markley JL (2007) The worldwide Protein Data Bank (wwPDB): ensuring a single uniform archive of PDB data. Nucleic Acids Res 35:D301–D303
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242
Word JM, Lovell SC, Richardson JS, Richardson DC (1999) Asparagine and glutamine: using hydrogen atom contacts in the choice of side-chain amide orientation. J Mol Biol 285:1735–1747
Weichenberger CX, Sippl MJ (2006) NQ-Flipper: validation and correction of asparagine/glutamine amide rotamers in protein crystal structures. Bioinformatics 22:1397–1398
Srinivasan J, Cheatham TE, Cieplak P, Kollman PA, Case DA (1998) Continuum solvent studies of the stability of DNA, RNA and phosphoramidate—DNA helices. J Am Chem Soc 120:9401–9409
Massova I, Kollman PA (1999) Computational alanine scanning to probe protein–protein interactions: a novel approach to evaluate binding free energies. J Am Chem Soc 121:8133–8143
Naim M, Bhat S, Rankin KN, Dennis S, Chowdhury SF, Siddiqi I, Drabik P, Sulea T, Bayly CI, Jakalian A, Purisima EO (2007) Solvated interaction energy (SIE) for scoring protein–ligand binding affinities. 1. exploring the parameter space. J Chem Inf Model 47:122–133
Kongsted J, Ryde U (2009) An improved method to predict the entropy term with the MM/PBSA approach. J Comput Aided Mol Des 23:63–71
Onufriev A, Bashford D, Case DA (2004) Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins 55:383–394
Lamm G, Szabo A (1986) Langevin modes of macromolecules. J Chem Phys 85:7334–7348
Kottalam J, Case DA (1990) Langevin modes of macromolecules—applications to Crambin and Dna hexamers. Biopolymers 29:1409–1421
Case DA (1994) Normal-mode analysis of protein dynamics. Curr Opin Struct Biol 4:285–290
Irwin JJ, Shoichet BK (2005) ZINC–a free database of commercially available compounds for virtual screening. J Chem Inf Model 45:177–182
Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M (2010) KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res 38:D355–D360
Heyer LJ, Kruglyak S, Yooseph S (1999) Exploring expression data: identification and analysis of coexpressed genes. Genome Res 9:1106–1115
Guha R, Howard MT, Hutchison GR, Murray-Rust P, Rzepa H, Steinbeck C, Wegner J, Willighagen EL (2006) The Blue Obelisk-interoperability in chemical informatics. J Chem Inf Model 46:991–998
Acknowledgments
We would like to thank Ulf Ryde for access to the programs changepdb and changecrd that facilitate the estimation of entropic contributions to the free energy of binding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lill, M.A., Danielson, M.L. Computer-aided drug design platform using PyMOL. J Comput Aided Mol Des 25, 13–19 (2011). https://doi.org/10.1007/s10822-010-9395-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-010-9395-8