Abstract
Purpose
To evaluate embryologic outcomes among paired IVF cycles in which a microfluidics chip was utilized compared to density gradient centrifugation for sperm processing.
Methods
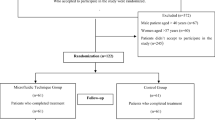
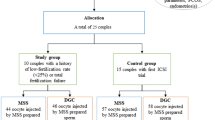
This was a retrospective cohort study of 88 paired IVF cycles from patients aged 18–44 years at a university-affiliated IVF center. Fresh cycles from patients undergoing ICSI with sperm processed by a microfluidics chamber (microfluidics cycles) were compared to the same patients’ previous ICSI cycles in which sperm was processed via density gradient centrifugation (control cycles). The primary outcome was the high-quality blastulation rate.
Results
High-quality blastulation rate per oocyte retrieved was significantly higher in the microfluidics group compared to the control group (21.1% versus 14.5%, p < 0.01) as was the blastulation rate per 2PN (42.7% versus 30.8%, p < 0.01). Fertilization rates were significantly higher in the microfluidics group. The euploidy rate per oocyte retrieved was significantly higher in the microfluidics group compared with the control group (8.5% versus 4.3%, p = 0.04), while the euploidy rate per embryo biopsied was comparable (32.6% versus 21.8%, p = 0.09). In patients with male factor infertility, the high-quality blastulation rate was similar between the control and microfluidics cycles. There was a significantly higher blastulation rate among microfluidics cycles in patients without a diagnosis of male factor infertility (p < 0.01).
Conclusion
In this study, several embryologic outcomes, including fertilization rate, high-quality blastulation rate, and euploidy rate, were significantly higher in the microfluidics group compared to the control group. Microfluidics sperm processing may be a way to improve embryologic outcomes.

Similar content being viewed by others
References
Chen M, Wong SL, Wu LL, Gordon YE, Heilbronn LK, Robker RL. Differential impacts of gonadotrophins, IVF and embryo culture on mouse blastocyst development. Reprod Biomed Online Elsevier. 2019;39:372–82.
Choi YH, Velez IC, Macías-García B, Riera FL, Ballard CS, Hinrichs K. Effect of clinically-related factors on in vitro blastocyst development after equine ICSI. Theriogenol Elsevier. 2016;85:1289–96.
Vaughan DA, Sakkas D. Sperm selection methods in the 21st century. Biol Reprod. 2019;101:1076–82.
Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006;12:608–15.
Mikwar M, MacFarlane AJ, Marchetti F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat Res Mutat Res Elsevier. 2020;785:108320.
Tomlinson M, Lewis S, Morroll D. Human Fertility an international, multidisciplinary journal dedicated to furthering research and promoting good practice Sperm quality and its relationship to natural and assisted conception: British Fertility Society Guidelines for practice. Hum Fertil. 2013;16:175–93.
Colaco S, Sakkas D. Paternal factors contributing to embryo quality. J Assist Reprod Genet. 2018;35:1953–68.
Arumugam M, Prashanth Shetty D, Shetty Kadandale J, Kumari NS. Association of sperm aneuploidy frequency and DNA fragmentation index in infertile men. J Reprod Infertil. 2019;20:121–6.
Marzano G, Chiriacò MS, Primiceri E, Dell’aquila ME, Ramalho-Santos J, Zara V, et al. Sperm selection in assisted reproduction: a review of established methods and cutting-edge possibilities. Biotechnol Adv. 2019;40
Henkel RR, Schill W-BB. Sperm preparation for ART. Reprod Biol Endocrinol. BioMed Central; 2003;1:1–22
Yalcinkaya Kalyan E, Can Celik S, Okan O, Akdeniz G, Karabulut S, Caliskan E. Does a microfluidic chip for sperm sorting have a positive add-on effect on laboratory and clinical outcomes of intracytoplasmic sperm injection cycles? A sibling oocyte study. Andrologia. 2019;51:1–6.
Parrella A, Choi D, Keating D, Rosenwaks Z, Palermo GD. A microfluidic device for selecting the most progressively motile spermatozoa yields a higher rate of euploid embryos. Fertil Steril Elsevier. 2018;110:e342.
Smith GD, Takayama S. Application of microfluidic technologies to human assisted reproduction. Mol Hum Reprod. 2017;23:257–68.
Oseguera-López I, Ruiz-Díaz S, Ramos-Ibeas P, Pérez-Cerezales S. Novel techniques of sperm selection for improving IVF and ICSI outcomes. Front Cell Dev Biol. 2019;7:1–23.
Quinn MM, Jalalian L, Ribeiro S, Ona K, Demirci U, Cedars MI, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33:1388–93.
McQueen DB, Zhang J, Robins JC. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2019;112:54–60.
Yetkinel S, Esra, Kilicdag B, Pinar, Aytac C, Haydardedeoglu B, et al. Effects of the microfluidic chip technique in sperm selection for intracytoplasmic sperm injection for unexplained infertility: a prospective randomized controlled trial. J Assist Reprod Genet. 2019;36:403–9.
Parrella A, Keating D, Cheung S, Xie P, Stewart JD, Rosenwaks Z, et al. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J Assist Reprod Genet. 2019;36:2057–66.
Diluigi AJ, Engmann L, Schmidt DW, Benadiva CA, Nulsen JC. A randomized trial of microdose leuprolide acetate protocol versus luteal phase ganirelix protocol in predicted poor responders. Fertil Steril Elsevier. 2011;95:2531–3.
Johnston-Macananny EB, Diluigi AJ, Engmann LL, Maier DB, Benadiva CA, Nulsen JC. Selection of first in vitro fertilization cycle stimulation protocol for good prognosis patients gonadotropin releasing hormone antagonist versus agonist protocols. J Reprod Med Obstet Gynecol. 2011;56:12–6.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–11.
Cooper TG, Noonan E, Von Eckardstein S, Auger J, Gordon Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45.
Anbari F, Ali M, Munaf A, Ahamed S, Mangoli E, Nabi A, et al. Microfluidic sperm selection yields higher sperm quality compared to conventional method in ICSI program: a pilot study. Syst Biol Reprod Med. 2021;67:137–43.
Keskin M, Pabuçcu EG, Arslanca T, Demirkıran ÖD, Pabuçcu R. Does microfluidic sperm sorting affect embryo euploidy rates in couples with high sperm DNA fragmentation? Reprod Sci. 2021;Published online ahead of print.
Pujol A, Garciá-Peiró A, Ribas-Maynou J, Lafuente R, Mataró D, Vassena R. A microfluidic sperm-sorting device reduces the proportion of sperm with double-stranded DNA fragmentation. Zygote. 2021;July:1–6.
Wald K, Hariton E, Morris JR, Chi EA, Jaswa EG, Cedars MI, et al. Changing stimulation protocol on repeat conventional ovarian stimulation cycles does not lead to improved laboratory outcomes. Fertil Steril Elsevier Inc. 2021;116:757–65.
Acknowledgements
We wish to thank all the physician, nursing, and embryology staff at the Center for Advanced Reproductive Services for their collaboration and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Godiwala, P., Almanza, E., Kwieraga, J. et al. Embryologic outcomes among patients using a microfluidics chip compared to density gradient centrifugation to process sperm: a paired analysis. J Assist Reprod Genet 39, 1523–1529 (2022). https://doi.org/10.1007/s10815-022-02504-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02504-1