Abstract
Purpose
To detect a possible bias in sperm DNA fragmentation (SDF) testing when performed on semen samples or on those few spermatozoa selected for Intracytoplasmic Sperm Injection (ICSI) treatments.
Methods
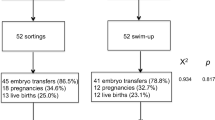
A multimethodological analysis of Single- and Double-Strand DNA Breaks (SSB and DSB, respectively) was performed through the Neutral Comet, the Alkaline Comet, the Sperm Chromatin Dispersion (SCD) and the Terminal deoxynucleotidyl transferase dUTP Nick End Labelling (TUNEL) assays. SDF was evaluated in (i) semen samples from 23 infertile patients (not achieving pregnancy or suffering recurrent miscarriage); (ii) samples after a Swim-up and (iii) spermatozoa microselected for ICSI (ICSI-S).
Results
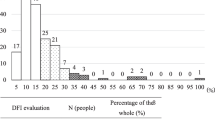
The analysis of 3217 ICSI-S revealed a significant reduction of SSB values compared to the Ejaculate and the Swim-up samples. On the contrary, DSB values were not reduced after any sperm selection method. The No-pregnancy group presented poorer semen parameters and higher SSB values. The Recurrent miscarriage group presented better semen parameters but also higher DSB values.
Conclusion
The analysis of SDF on semen samples may not be fully representative of those few spermatozoa selected for ICSI. Since oxidative stress impairs sperm motility and causes SSB, selecting a motile sperm may intrinsically imply choosing a sperm not affected by this damage. DSB have an enzymatic origin which does not affect motility, making it difficult to select a sperm without this damage. Therefore, ICSI treatments could be effective in patients presenting high SSB values. Patients presenting high DSB values should expect bad ICSI results if this damage is not reduced through other specific methods.


Similar content being viewed by others
Code availability
Not applicable.
References
Vaughan DA, Sakkas D. Sperm selection methods in the 21st century. Biol Reprod. 2019;101:1076–82.
Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:1–9.
Jeyendran RS, Caroppo E, Rouen A, Anderson A, Puscheck E. Selecting the most competent sperm for assisted reproductive technologies. Fertil Steril. 2019;111:851–63.
Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331–45.
Ioannou D, Griffin DK. Male fertility, chromosome abnormalities, and nuclear organization. Cytogenet Genome Res. 2011;133:269–79.
Hamada A, Esteves SC, Agarwal A. Genetics and male infertility. Androl Clin. 2006:462–80.
Dhanoa JK, Mukhopadhyay CS, Arora JS. Y-chromosomal genes affecting male fertility: a review. Vet World. 2016;9:783–91.
Simon L, Emery BR, Carrell DT. Sperm DNA fragmentation consequences for reproduction. Adv Exp Med Biol. 2019;1166.
Oliva R. Protamines and male infertility. Hum Reprod Update. 2006;12:417–35.
Gunes S, Arslan MA, Hekim GNT, Asci R. The role of epigenetics in idiopathic male infertility. J Assist Reprod Genet. 2016;33:553–69.
Samplaski MK, Agarwal A, Sharma R, Sabanegh E. New generation of diagnostic tests for infertility: review of specialized semen tests. Int J Urol. 2010;17:839–47.
Katz DJ, Teloken P, Shoshany O. Male infertility - the other side of the equation. Aust Fam Physician. 2017;46:641–6.
Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–7.
Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40:443–53.
Agarwal A, Allamaneni SSR. Sperm DNA damage assessment: a test whose time has come. Fertil Steril. 2005;84:850–3.
Celik-Ozenci C, Jakab A, Kovacs T, Catalanotti J, Demir R, Bray-Ward P, et al. Sperm selection for ICSI: shape properties do not predict the absence or presence of numerical chromosomal aberrations. Hum Reprod. 2004;19:2052–9.
Avendaño C, Franchi A, Taylor S, Morshedi M, Bocca S, Oehninger S. Fragmentation of DNA in morphologically normal human spermatozoa. Fertil Steril. 2009;91:1077–84.
Majzoub A, Agarwal A, Esteves SC. Clinical utility of sperm DNA damage in male infertility. Panminerva Med. 2019;61:118–27.
Panner Selvam MK, Agarwal A. A systematic review on sperm DNA fragmentation in male factor infertility: Laboratory assessment. Arab J Urol. 2018;16:65–76.
Bach PV, Schlegel PN. Sperm DNA damage and its role in IVF and ICSI. Basic Clin Androl. 2016;26:1–10.
Agarwal A, Barbăroșie C, Ambar R, Finelli R. The impact of single- and double-strand DNA breaks in human spermatozoa on assisted reproduction. Int J Mol Sci. 2020;21:1–14.
Ribas-Maynou J, García-Peiró A, Abad C, Amengual MJ, Navarro J, Benet J. Alkaline and neutral Comet assay profiles of sperm DNA damage in clinical groups. Hum Reprod. 2012;27:652–8.
Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85.
Alkhayal A, San Gabriel M, Zeidan K, Alrabeeah K, Noel D, McGraw R, et al. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet. 2013;30:1519–24.
Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9.
Simon L, Proutski I, Stevenson M, Jennings D, McManus J, Lutton D, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod BioMed Online. 2013;26:68–78.
Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod BioMed Online. 2015;30:120–7.
Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: Systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8.
Simon L, Zini A, Dyachenko A, Ciampi A, Carrell D. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 2017;19:80–90.
Yang H, Li G, Jin H, Guo Y, Sun Y. The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl Androl Urol. 2019;8:356–65.
Anifandis G, Bounartzi T, Messini CI, Dafopoulos K, Markandona R, Sotiriou S, et al. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia. 2015;47:295–302.
The practice committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99:673–7.
Avendaño C, Oehninger S. DNA fragmentation in morphologically normal spermatozoa: how much should we be concerned in the ICSI era? J Androl. 2011;32:356–63.
WHO. WHO laboratory manual for the examination and processing of human semen. World Heal Organ Press 2010; 5th Editio.
Balaban B, Sakkas D, Gardner DK. Laboratory procedures for human in vitro fertilization. Semin Reprod Med. 2014;32:272–82.
Ribas-Maynou J, García-Peiró A, Fernandez-Encinas A, Amengual M, Prada E, Cortés P, et al. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS One. 2012;7:e44679.
Fernández JL, Muriel L, Goyanes V, Segrelles E, Gosálvez J, Enciso M, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Am Soc Reprod Med. 2005;84:833–42.
Ribas-Maynou J, García-Peiró A, Fernández-Encinas A, Abad C, Amengual MJ, Prada E, et al. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology. 2013;1:715–22.
García-Peiró A, Oliver-Bonet M, Navarro J, Abad C, Guitart M, Amengual MJ, et al. Dynamics of sperm DNA fragmentation in patients carrying structurally rearranged chromosomes. Int J Androl. 2011;34:e546–53.
Gosálvez J, Caballero P, López-Fernández C, Ortega L, Guijarro JA, Fernández JL, et al. Can DNA fragmentation of neat or swim-up spermatozoa be used to predict pregnancy following ICSI of fertile oocyte donors? Asian J Androl. 2013;15:812–8.
Oguz Y, Guler I, Erdem A, Mutlu MF, Gumuslu S, Oktem M, et al. The effect of swim-up and gradient sperm preparation techniques on deoxyribonucleic acid (DNA) fragmentation in subfertile patients. J Assist Reprod Genet. 2018;35:1083–9.
Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1.
Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, et al. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod BioMed Online. 2003;7:477–84.
Agarwal A, Sharma R, Harlev A, Esteves SC. Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: a systematic review and meta-analysis. Asian J Androl. 2016;18:163–70.
Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007;70:532–8.
Lara-Cerrillo S, Gual-Frau J, Benet J, Abad C, Prats J, Amengual MJ, et al. Microsurgical varicocelectomy effect on sperm telomere length, DNA fragmentation and seminal parameters. Hum Fertil. 2020;9:1–7.
Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril 2014; 102:998-1005.e8.
Simon L, Lutton D, McManus J, Lewis SEM. Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril. 2011;95:652–7.
Chi H, Kim S, Kim Y, Park J, Yoo C, Park I, et al. ICSI significantly improved the pregnancy rate of patients with a high sperm DNA fragmentation index. Clin Exp Reprod Med. 2017;44:132–40.
Coban O, Serdarogullari M, Yarkiner Z, Serakinci N. Investigating the level of DNA double-strand break in human spermatozoa and its relation to semen characteristics and IVF outcome using phospho-histone H2AX antibody as a biomarker. Andrology. 2019:1–6.
Paiano J, Wu W, Yamada S, Sciascia N, Callen E, Paola Cotrim A, et al. ATM and PRDM9 regulate SPO11-bound recombination intermediates during meiosis. Nat Commun. 2020;11:1–15.
Lange J, Pan J, Cole F, Thelen MP, Jasin M, Keeney S. ATM controls meiotic double-strand-break formation. Nature. 2011;479:237–40.
Keeney S, Lange J, Mohibullah N. Self-organization of meiotic recombination initiation: general principles and molecular pathways. Annu Rev Genet. 2014;48:187–214.
García-Rodríguez A, Gosálvez J, Agarwal A, Roy R, Johnston S. DNA damage and repair in human reproductive cells. Int J Mol Sci. 2019;20:1–22.
Casanovas A, Ribas-Maynou J, Lara-Cerrillo S, Jimenez-Macedo AR, Hortal O, Benet J, et al. Double-stranded sperm DNA damage is a cause of delay in embryo development and can impair implantation rates. Fertil Steril. 2019;111:699–707.e1.
Green KA, Patounakis G, Dougherty MP, Werner MD, Scott RT, Franasiak JM. Sperm DNA fragmentation on the day of fertilization is not associated with embryologic or clinical outcomes after IVF/ICSI. J Assist Reprod Genet. 2020;37:71–6.
Gunes S, Sertyel S. Sperm DNA damage and oocyte repair capability. A Clin Guid to Sperm DNA Chromatin Damage. 2018:321–46.
Eisenberg ML, Sapra KJ, Kim SD, Chen Z, Buck Louis GM. Semen quality and pregnancy loss in a contemporary cohort of couples recruited before conception: Data from the LIFE Study. Fertil Steril. 2017;108:613–9.
Marzano G, Chiriacò MS, Primiceri E, Dell’Aquila ME, Ramalho-Santos J, Zara V, et al. Sperm selection in assisted reproduction: a review of established methods and cutting-edge possibilities. Biotechnol Adv. 2020;40:107498.
Quinn MM, Jalalian L, Ribeiro S, Ona K, Demirci U, Cedars MI, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33:1388–93.
Xiao S, Riordon J, Simchi M, Lagunov A, Hannam T, Jarvi K, et al. FertDish: microfluidic sperm selection-in-a-dish for intracytoplasmic sperm injection. Lab Chip. 2021;21:775–83.
Parrella A, Keating D, Cheung S, Xie P, Stewart JD, Rosenwaks Z, et al. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J Assist Reprod Genet. 2019;36:2057–66.
Smith GD, Takayama S. Application of microfluidic technologies to human assisted reproduction. Mol Hum Reprod. 2017;23:257–68.
Samuel R, Feng H, Jafek A, Despain D, Jenkins T, Gale B. Microfluidic-based sperm sorting & analysis for treatment of male infertility. Transl Androl Urol. 2018;7:S336–47.
Anbari F, Khalili MA, Sultan Ahamed AM, Mangoli E, Nabi A, Dehghanpour F, et al. Microfluidic sperm selection yields higher sperm quality compared to conventional method in ICSI program: a pilot study. Syst Biol Reprod Med. 2021;00:1–7.
Palermo GD, O’Neill CL, Chow S, Cheung S, Parrella A, Pereira N, et al. Intracytoplasmic sperm injection: State of the art in humans. Reproduction. 2017;154:F93–F110.
Franco J, Baruffi R, Mauri A, Petersen C, Oliveira J, Vagnini L. Significance of large nuclear vacuoles in human spermatozoa: implications for ICSI. Reprod BioMed Online. 2008;17:42–5.
Gosálvez J, Migueles B, López-Fernández C, Sanchéz-Martín F, Sáchez-Martín P. Single sperm selection and DNA fragmentation analysis: the case of MSOME/IMSI. Nat Sci. 2013;05:7–14.
Pastuszek E, Kiewisz J, Skowronska P, Liss J, Lukaszuk M, Bruszczynska A, et al. An investigation of the potential effect of sperm nuclear vacuoles in human spermatozoa on DNA fragmentation using a neutral and alkaline Comet assay. Andrology. 2017;5:392–8.
Hammoud I, Boitrelle F, Ferfouri F, Vialard F, Bergere M, Wainer B, et al. Selection of normal spermatozoa with a vacuole-free head (x6300) improves selection of spermatozoa with intact DNA in patients with high sperm DNA fragmentation rates. Andrologia. 2013;45:163–70.
Luna D, Hilario R, Dueñas-Chacón J, Romero R, Zavala P, Villegas L, et al. The IMSI procedure improves laboratory and clinical outcomes without compromising the aneuploidy rate when compared to the classical ICSI procedure. Clin Med Insights Reprod Heal. 2015;9:CMRH.S33032.
Wilding M, Coppola G, Di Matteo L, Palagiano A, Fusco E, Dale B. Intracytoplasmic injection of morphologically selected spermatozoa (IMSI) improves outcome after assisted reproduction by deselecting physiologically poor quality spermatozoa. J Assist Reprod Genet. 2011;28:253–62.
Duran-Retamal M, Morris G, Achilli C, Gaunt M, Theodorou E, Saab W, et al. Live birth and miscarriage rate following intracytoplasmic morphologically selected sperm injection vs intracytoplasmic sperm injection: an updated systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2019:1–10.
Setti AS, De Almeida Ferreira Braga DP, Iaconelli A, Aoki T, Borges E. Twelve years of MSOME and IMSI: a review. Reprod BioMed Online. 2013;27:338–52.
Teixeira D, Barbosa M, Ferriani R, Navarro P, Nastri C. Regular ( ICSI ) versus ultra-high magnification ( IMSI ) sperm selection for assisted reproduction ( Review ). Cochrane Libr. 2013.
López G, Lafuente R, Checa MA, Carreras R, Brassesco M. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J Androl. 2013;15:790–4.
Simopoulou M, Sfakianoudis K, Antoniou N, Maziotis E, Rapani A, Bakas P, et al. Making IVF more effective through the evolution of prediction models: is prognosis the missing piece of the puzzle? Syst Biol Reprod Med. 2018;64:305–23.
Bounartzi T, Dafopoulos K, Anifandis G, Messini CI, Koutsonikou C, Kouris S, et al. Pregnancy prediction by free sperm DNA and sperm DNA fragmentation in semen specimens of IVF/ICSI-ET patients. Hum Fertil. 2016;19:56–62.
Aknowledgements
We thank Dr. Denny Sakkas for his useful comments on the final manuscript.
Funding
SLC received an Industrial Doctorate grant (Doctorados Industriales) given by the Economy, Industry and Competitivity Ministry of Spain (Ministerio de Economía, Industria y Competitividad) and the State Investigation Agency (Agencia Estatal de Investigación) (Ref.: DI-16-08429).
Author information
Authors and Affiliations
Contributions
SLC performed the experiments and participated in the study design, the data analysis and the manuscript writing and review. JRM participated in the study design and data analysis. TLR and CRI performed the experiments. JB contributed to the manuscript review. AGP contributed to the study design, analysis of data and manuscript review and final approval.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Corporació Sanitaria Parc Taulí Ethics Committee (Ref.: 2014676) and signed informed consents were obtained from all patients.
Consent to participate
Patients consented to participate.
Consent for publication
Patients consented to participate.
Conflict of interest
AGP is the director of the CIMAB centre, a university spin-out company with a commercial interest in SDF testing. No other author has a conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lara-Cerrillo, S., Ribas-Maynou, J., Rosado-Iglesias, C. et al. Sperm selection during ICSI treatments reduces single- but not double-strand DNA break values compared to the semen sample. J Assist Reprod Genet 38, 1187–1196 (2021). https://doi.org/10.1007/s10815-021-02129-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02129-w