Abstract
Purpose
The study aimed to describe the ultrastructure of two human mature oocyte intracytoplasmic dysmorphisms, the bull-eye inclusion and the granular vacuole, with evaluation of clinical outcomes after intracytoplasmic sperm injection (ICSI) treatment.
Methods
We retrospectively evaluated 4099 consecutive ICSI cycles during the period 2003–2013. Three groups were compared: controls, those with a bulls-eye inclusion, and those with granular vacuoles. Oocyte dysmorphisms were evaluated by transmission electron microscopy and in situ fluorescence hybridization (FISH). Detailed data on demographic and stimulation characteristics, as well as on embryological, clinical, and newborn outcomes, are fully presented.
Results
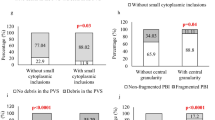
The bull-eye inclusion is a prominent smooth round structure containing trapped vesicles, being surrounded by lipid droplets. The presence of this dysmorphism in the oocyte cohort had no clinical impact except when transferred embryos were exclusively derived from dysmorphism oocytes. The granular vacuole is delimited by a discontinuous double membrane and contains lipid droplets and vesicles. As FISH analysis revealed the presence of chromosomes, they probably represent pyknotic nuclei. The presence of this dysmorphism in the oocyte cohort had no clinical impact except when at least one transferred embryo was derived from dimorphic oocytes.
Conclusions
Poor clinical outcomes were observed with transfer of embryos derived from dysmorphism oocytes, although without causing gestation or newborn problems. The bull-eye inclusion and granular vacuoles may thus be new prognostic factors for clinical outcomes.






Similar content being viewed by others
References
Van Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Elect Microsc Techn. 1990;16:324–46.
Van Blerkom J, Henry G. Oocyte dysmorphism and aneuploidy in meiotically mature human oocytes after ovarian stimulation. Hum Reprod. 1992;7:379–90.
Magli NC, Jones GM, Lundi K, Van den Abbeel E, The ESHRE Special Interest Group on Embryology. Atlas of human embryology: from oocytes to preimplantation embryos. Hum Reprod. 2012;27 Suppl 1:1–91.
Sundström P, Nilsson BO. Meiotic and cytoplasmic maturation of oocytes collected in stimulated cycles is asynchronous. Hum Reprod. 1988;3:613–9.
Ebner T, Moser M, Sommergruber M, Tews G. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development. Hum Reprod Update. 2003;9:251–62.
Ebner T, Moser M, Tews G. Is oocyte morphology prognostic of embryo developmental potential after ICSI? Reprod BioMed Online. 2006;12:507–12.
Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17:34–45.
Keef D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential. Fertil Steril. 2015;103:317–22.
Ebner T, Balaban B, Moser M, Shebl O, Urman B, Ata B, et al. Automatic user-independent zona pellucida imaging at the oocyte stage allows for the prediction of preimplantation development. Fertil Steril. 2010;94:913–20.
Shi W, Xu B, Wu L-M, Jin R-T, Luan H-B, Luo L-H, et al. Oocytes with a dark zona pellucida demonstrate lower fertilization, implantation and clinical pregnancy rates in IVF/ICSI cycles. PLoS One. 2014;9:e89409. doi:10.1371/journal.pone.0089409.
Sauerbrun-Cutler M-T, Vega M, Breborowicz A, Gonzales E, Stein D, Lederman M, et al. Oocyte zona pellucida dysmorphology is associated with diminished in-vitro fertilization success. J Ovarian Res. 2015;8:5. doi:10.1186/s13048-014-0111-5.
Sousa M, Teixeira da Silva J, Silva J, Cunha M, Viana P, Oliveira E, et al. Embryological, clinical and ultrastructural study of human oocytes presenting indented zona pellucida. Zygote. 2015;23:145–57.
Ebner T, Moser M, Yaman C, Feichtinger O, Hartl J, Tews G. Elective transfer of embryos selected on the basis of first polar body morphology is associated with increased rates of implantation and pregnancy. Fertil Steril. 1999;72:599–603.
Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, Tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod. 2000;15:427–30.
Cupisti S, Conn CM, Fragouli E, Whalley K, Mills JS, Faed MJW, et al. Sequential FISH analysis of oocytes and polar bodies reveals aneuploidy mechanisms. Prenat Diagn. 2003;23:663–8.
Geraedts J, Montag M, Magli MC, Repping S, Handyside A, Staessen C, et al. Polar body array CGH for prediction of the status of the corresponding oocyte. Part I: clinical results. Hum Reprod. 2011;26:3173–80.
Schmutzler AG, Acar-Perk B, Weimer J, Salmassi A, Sievers K, Tobler M, et al. Oocyte morphology on day 0 correlates with aneuploidy as detected by polar body biopsy and FISH. Arch Gynecol Obstet. 2014;289:445–50.
Zhou W, Fu L, Sha W, Chu D, Li Y. Relationship of polar bodies morphology to embryo quality and pregnancy outcome. Zygote. 2015. doi:10.1017/S0967199415000325.
Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, et al. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003;18:1289–93.
Petersen CG, Oliveira JBA, Mauri AL, Massaro FC, Baruffi RLR, Pontes A, et al. Relationship between visualization of meiotic spindle in human oocytes and ICSI outcomes: a meta-analysis. Reprod BioMed Online. 2009;18:235–43.
Korkmaz C, Tekin YB, Sakinci M, Ercan CM. Effects of maternal ageing on ICSI outcomes and embryo development in relation to oocytes morphological characteristics of birefringent structures. Zygote. 2015;23:550–5.
Ménézo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18:357–65.
Ebner T, Moser M, Yaman C, Sommergruber M, Hartl J, Jesacher K, et al. Prospective hatching of embryos developed from oocytes exhibiting difficult oolemma penetration during ICSI. Hum Reprod. 2002;17:1317–20.
Ebner T, Moser M, Sommergruber M, Puchner M, Wiesinger R, Tews G. Developmental competence of oocytes showing increased cytoplasmic viscosity. Hum Reprod. 2003;18:1294–8.
Ebner T, Shebl O, Moser M, Sommergruber M, Tews G. Developmental fate of ovoid oocytes. Hum Reprod. 2008;23:62–6.
Machtinger R, Politch JA, Hornstein MD, Ginsburg ES, Racowsky C. A giant oocyte in a cohort of retrieved oocytes: does it have any effect on the in vitro fertilization cycle outcome? Fertil Steril. 2011;95:573–6.
Sousa M, Barros A, Silva J, Tesarik J. Developmental changes in calcium contents of ultrastructurally distinct subcellular compartments of preimplantation embryos. Mol Hum Reprod. 1997;3:83–90.
Wilding M, Dale B, Marino M, di Matteo, Alviggi C, Pisaturo ML, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–17.
Wells D, Bermúdez MG, Steuerwald N, Malter HE, Thornhill AR, Cohen J. Association of abnormal morphology and altered gene expression in human preimplantation embryos. Fertil Steril. 2005;84:343–55.
Gasca S, Pellestor F, Assou S, Loup V, Anahory T, Dechaud H, et al. Identifying new human oocyte marker genes: a microarray approach. Reprod BioMed Online. 2007;14:175–83.
Grøndahl ML, Andersen CY, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod. 2010;25:957–68.
Kakourou G, Jaroudi S, Tulay P, Heath C, Serhal P, Haper JC, et al. Investigation of gene expression profiles before and after embryonic genome activation and assessment of functional pathways at the human metaphase II oocyte and blastocyst stage. Fertil Steril. 2013;99:803–14.
Alikani M, Palermo G, Adler A, Bertoli M, Blake M, Cohen J. Intracytoplasmic sperm injection in dysmorphic human oocytes. Zygote. 1995;3:283–8.
De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1996;11:595–7.
Sherhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:1267–70.
Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization and embryo quality. Hum Reprod. 1997;12:1750–5.
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3431–3.
Loutradis D, Drakakis P, Kallianidis K, Milingos S, Dendrinos S, Michalas S. Oocyte morphology correlates with embryo quality and pregnancy rate after intracytoplasmic sperm injection. Fertil Steril. 1999;72:240–4.
Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, et al. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod Biomed Online. 2006;13:661–7.
Yakin K, Balaban B, Isiklar A, Urman B. Oocyte dysmophism is not associated with aneuploidy in the developing embryo. Fertil Steril. 2007;88:811–6.
Figueira RCS, Braga DPAF, Semião-Francisco L, Madaschi C, Iaconelli Jr A, Borges Jr E. Metaphase II human oocyte morphology: contributing factors and effects on fertilization potential and embryo developmental ability in ICSI cycles. Fertil Steril. 2010;94:1115–7.
Braga DPAF, Setti AS, Figueira RCS, Machado RB, Iaconelli Jr A, Borges Jr E. Influence of oocyte dysmorphisms on blastocyst formation and quality. Fertil Steril. 2013;100:748–54.
Ashrafi M, Karimian L, Eftekhari-Yazdi P, Hasani F, Arabipoor A, Bahmanabadi A, et al. Effect of oocyte dysmorphisms on intracytoplasmic sperm injection cycle outcomes in normal ovarian responders. J Obstet Gynaecol Res. 2015;41:1912–20.
Yu EJ, Ahn H, Lee JM, Jee BC, Kim SH. Fertilization and embryo quality of mature oocytes with specific morphological abnormalities. Clin Exp Reprod Med. 2015;42:156–62.
Otsuki J, Nagai Y, Chiba K. Lipofuscin bodies in human oocytes as an indicator of oocyte quality. J Assist Reprod Genet. 2007;24:263–70.
Kahraman S, Yakin K, Dönmez E, Samli H, Bahçe M, Cengiz G, et al. Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum Reprod. 2000;15:2390–3.
Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–6.
Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Shebl O, Jesacher K, et al. Occurrence and developmental consequences of vacuoles throughout preimplantation development. Fertil Steril. 2005;83:1635–40.
Bianchi V, Macchiarelli G, Borini A, Lappi M, Cecconi S, Miglietta S, et al. Fine morphological assessment of quality of human mature oocytes after slow freezing or vitrification with a closed device: a comparative analysis. Reprod Biol Endocrinol. 2014;12:110. doi:10.1186/1477-7827-12-110.
Palmerini MG, Antinori M, Maione M, Cerusico F, Versaci C, Nottola SA, et al. Ultrastructure of immature and mature human oocytes after cryotop vitrification. J Reprod Dev. 2014;60:411–20.
Bianchi S, Macchiarelli G, Micara G, Linari A, Boninsegna C, Aragona C, et al. Ultrastructural markers of quality are impaired in human metaphase II aged oocytes: a comparison between reproductive and in vitro aging. J Assist Reprod Genet. 2015;32:1343–58.
Fancsovits P, Murber A, Gilán ZT, Rigó Jr J, Urbancsek J. Human oocytes containing large cytoplasmic vacuoles can result in pregnancy and viable offspring. Reprod BioMed Online. 2011;23:513–6.
Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–7.
Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod BioMed Online. 2008;16:113–8.
Akarsu C, Çağlar G, Vicdan K, Sözen E, Biberoğlu K. Smooth endoplasmic reticulum aggregations in all retrieved oocytes causing recurrent multiple anomalies: case report. Fertil Steril. 2009;92(1496):e1–3.
Sá R, Cunha M, Silva J, Luís A, Oliveira C, Teixeira da Silva J, et al. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril. 2011;96:143–9.
Mateizel I, Van Landuyt L, Tournaye H, Verheyen G. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013;28:2111–7.
Hattori H, Nakamura Y, Nakajo Y, Araki Y, Kyono K. Deliveries of babies with normal health derived from oocytes with smooth endoplasmic reticulum clusters. J Assist Reprod Genet. 2014;31:1461–7.
Shaw-Jackson C, Van Beirs N, Thomas A-L, Rozenberg S, Autin C. Can healthy babies originate from oocytes with smooth endoplasmic reticulum aggregates? A systematic mini-review. Hum Reprod. 2014;29:1380–6.
Restelli L, Noci SD, Mangiarini A, Ferrari S, Somigliana E, Paffoni A. The impact of Alpha/ESHRE consensus regarding oocytes with aggregates of smooth endoplasmic reticulum (SERa) on in vitro fertilization outcome. J Assist Reprod Genet. 2015;32:1629–35.
Van Beirs N, Shaw-Jackson C, Rozenberg S, Autin C. Policy of IVF centres towards oocytes affected by smooth endoplasmic reticulum aggregates: a multicentric survey study. J Assist Reprod Genet. 2015;32:945–50.
Rooney DE, Czepulkowski BH. Human chromosome preparation. Essential techniques. UK: John Wiley & Sons Ltd; 1997 (Series Editor: B Rickwood).
Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF? Hum Reprod. 2007;22:2805–13.
Pinto F, Oliveira C, Cardoso MF, Teixeira da Silva J, Silva J, Sousa M, et al. Impact of GnRH ovarian stimulation protocols on intracytoplasmic sperm injection outcomes. Reprod Biol Endocrinol. 2009;7:5. doi:10.1186/1477-7827-7-5.
Tesarik J, Sousa M. Key elements of a highly efficient intracytoplasmic sperm injection technique: Ca2+ fluxes and oocyte cytoplasmic dislocation. Fertil Steril. 1995;64:770–6.
Vandervorst M, Liebaers I, Sermon K, Staessen C, De Vos A, Van de Velde H, et al. Successful preimplantation genetic diagnosis is related to the number of available cumulus-oocyte complexes. Hum Reprod. 1998;13:3169–76.
Gardner DK, Lane ML, Stevens J, Schlenker T, Schollcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8.
Radesic B, Tremellen K. Oocyte maturation employing a GnRH agonist in combination with low-dose hCG luteal rescue minimizes the severity of ovarian hyperstimulation syndrome while maintaining excellent pregnancy rates. Hum Reprod. 2011;26:3437–42.
Sousa M, Tesarik J. Ultrastructural analysis of fertilization failure after intracytoplasmic sperm injection. Hum Reprod. 1994;9:2374–80.
El Shafie M, Sousa M, Windt M-L, Kruger TF. An atlas of the ultrastructure of human oocytes. A guide for assisted reproduction. NY, USA: The Parthenon Publishing Group; 2000.
Coonen E, Dumoulin JCM, Ramaekers FCS, Hopman AHN. Optimal preparation of preimplantation embryo interphase nuclei for analysis by fluorescence in-situ hybridization. Hum Reprod. 1994;9:533–7.
Harper JC, Coonen E, Ramaekers FCS, Delhanty JDA, Handyside AH, Wilston RML, et al. Identification of the sex of human preimplantation embryos in two hours using an improved spreading method and fluorescent in-situ hybridization (FISH) using directly labelled probes. Hum Reprod. 1994;9:721–4.
Alves C, Sousa M, Silva J, Barros A. Preimplantation genetic diagnosis using FISH for carriers of Robertsonian translocations: the Portuguese experience. Prenat Diagn. 2002;22:1153–62.
Turnpenny P, Ellard S. Emery’s elements of medical genetics. Philadelphia, USA: Elsevier, Churchill Livingstone; 2012.
Forabosco A, Percesepe A, Santucci S. Incidence of non-age-dependent chromosomal abnormalities: a population-based study on 88965 amniocenteses. Eur J Hum Genet. 2009;17:897–903.
Papanikolaou EG, Vernaeve V, Kolibianakis E, Van Assche E, Bonduelle M, Liebaers I, et al. Is chromosome analysis mandatory in the initial investigation of normovulatory women seeking infertility treatment? Hum Reprod. 2005;20:2899–903.
Shevell T, Malone FD, Vidaver J, Porter TF, Luthy DA, Comstock CH, et al. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106:1039–45.
Ravel C, Berthaut I, Bresson JL, Siffroi JP, et al. Prevalence of chromosomal abnormalities in phenotypically normal and fertile adult males: large-scale survey of over 10000 sperm donor karyotypes. Hum Reprod. 2006;21:1484–89.
Clementini E, Palka C, Iezzi L, Stuppia L, Guanciali-Franchi P, Tiboni GM. Prevalence of chromosomal abnormalities in 2078 infertile couples referred for assisted reproductive techniques. Hum Reprod. 2005;20:437–42.
Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C. Male infertility: role of genetic background. Reprod BioMed Online. 2007;14:734–45.
Gekas J, Thepot F, Turleau C, Siffroi JP, Dadoune JP, Wasels R, et al. Chromosomal factors of infertility in candidate couples for ICSI: an equal risk of constitutional aberrations in women and men. Hum Reprod. 2001;16:82–90.
Schreurs A, Legius E, Meuleman C, Fryns J-P, D’Hooghe TM. Increased frequency of chromosomal abnormalities in female partners of couples undergoing in vitro fertilization or intracytoplasmic sperm injection. Fertile Steril. 2000;74:94–6.
De Sutter P, Stadhoufers R, Dutré M, Gerris J, Dhont M. Prevalence of chromosomal abnormalities and timing of karyotype analysis in patients with recurrent implantation failure (RIF) following assisted reproduction. FVV ObGyn. 2012;4:59–65.
Sousa M, Barros A, Tesarik J. Developmental changes in calcium dynamics, protein kinase C distributions and endoplasmic reticulum organization in human preimplantation embryos. Mol Hum Reprod. 1996;2:967–77.
Norppa H, Falck GC-M. What do human micronuclei contain? Mutagenesis. 2003;18:221–33.
Shimizu N. Molecular mechanisms of the origin of micronuclei from extrachromosomal elements. Mutagenesis. 2011;26:119–23.
Mantikou E, Wong KM, Repping S, Masteenbroek S. Molecular origin of mitotic aneuploidies in preimplantation embryos. Biochem Biophy Acta. 1822;2012:1921–30.
Nottola SA, Macchiarelli G, Familiari G. Fine structural markers of human oocyte quality in assisted reproduction. Austin J Reprod Med Infertil. 2014;1:5.
Acknowledgments
The authors would like to that the following persons: for oocyte retrieval to Jorge Beires, MD, PhD, Specialist in Gynecology and Obstetrics, from the Department of Gynecology and Obstetrics, Director of the Unit of Gynecology and Reproductive Medicine, Hospital of S. John, E.P.E., Porto, Portugal and José Manuel Teixeira da Silva, MD, Specialist in Gynecology and Obstetrics; for anesthesiology to José Correia, MD, Anesthetist (Department of Anesthesiology, Hospital of S. John, E.P.E., Porto, Portugal); for IVF laboratorial work assistance to Paulo Viana, MSc., Biologist, Clinical Embryologist and Nuno Barros MSc., Microbiologist (CGR-ABarros); for spermiology laboratorial work to Ana Gonçalves, MSc., Biochemist and Cláudia Osório MSc., Biologist (CGR-ABarros); for technical support on FISH analysis to Ana Raquel Azevedo, Ph.D. (ICBAS-UP); and for transmission electron microscopy technical support to Célia Soares, M.D. and Ângela Alves, BSc., Technical assistant for teaching and research (ICBAS-UP).
We also would like to thank all colleagues from the many other research articles, reviews, book chapters, and books that we did not cite in this manuscript but whose lecture was fundamental to the writing of the present manuscript.
Funding
UMIB is funded by National Funds through FCT-Foundation for Science and Technology, under the Pest-OE/SAU/UI0215/2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have followed all the rules of ethical conduct regarding originality, data processing and analysis, duplicate publication, and patient treatments.
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Capsule Poor clinical outcomes were observed with mixed or pure transfer of embryos derived from oocytes containing bull-eye inclusions or granular-vacuoles. These dimorphisms may be new prognostic factors for clinical outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 26 kb)
Rights and permissions
About this article
Cite this article
Sousa, M., Cunha, M., Silva, J. et al. Ultrastructural and cytogenetic analyses of mature human oocyte dysmorphisms with respect to clinical outcomes. J Assist Reprod Genet 33, 1041–1057 (2016). https://doi.org/10.1007/s10815-016-0739-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0739-8