Abstract
Purpose
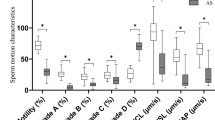
Asthenozoospermia is a common cause of human male infertility characterized by reduced sperm motility. The molecular mechanism that impairs sperm motility is not fully understood. This study proposed to identify novel biomarkers by focusing on sperm tail proteomic analysis of asthenozoospermic patients.
Methods
Sperm were isolated from normozoospermic and asthenozoospermic semen samples. Tail fractions were obtained by sonication followed by Percoll gradient. The proteins were extracted by solubilization and subjected to two-dimensional gel electrophoresis (2-DE); then, the spots were analyzed using Image Master 2D Platinum software. The significantly increased/decreased amounts of proteins in the two groups were exploited by matrix-assisted laser desorption-ionization time-of-flight/time-of-flight (MALDI-TOF-TOF) mass spectrometry.
Results
Three hundred ninety protein spots were detected in both groups. Twenty-one protein spots that had significantly altered amounts (p < 0.05) were excised and exploited using MALDI-TOF-TOF mass spectrometry. They led to the identification of the following 14 unique proteins: Tubulin beta 2B; glutathione S-transferase Mu 3; keratin, type II cytoskeletal 1; outer dense fiber protein 2; voltage-dependent anion-selective channel protein 2; A-kinase anchor protein 4; cytochrome c oxidase subunit 6B; sperm protein associated with the nucleus on the X chromosome B; phospholipid hydroperoxide glutathione peroxidase-mitochondrial; isoaspartyl peptidase/L-asparaginase; heat shock-related 70 kDa protein 2; stress-70 protein, mitochondrial; glyceraldehyde-3-phosphate dehydrogenase, testis-specific and clusterin.
Conclusion
Fourteen proteins present in different amounts in asthenozoospermic sperm tail samples were identified, four of which are reported here for the first time. These proteins might be used as markers for the better diagnosis of sperm dysfunctions, targets for male contraceptive development, and to predict embryo quality.



Similar content being viewed by others
References
Moore FL, Reijo-Pera RA. Male sperm motility dictated by mother’s mtDNA. Am J Hum Genet. 2000;67(3):543.
Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril. 1991;56(2):192–3.
Turner RM. Moving to the beat: a review of mammalian sperm motility regulation. Reprod Fertil Dev. 2005;18(2):25–38.
Pixton KL, Deeks ED, Flesch FM, Moseley FLC, Björndahl L, Ashton PR, et al. Sperm proteome mapping of a patient who experienced failed fertilization at IVF reveals altered expression of at least 20 proteins compared with fertile donors: case report. Hum Reprod. 2004;19(6):1438–47.
Ergur AR, Dokras A, Giraldo JL, Habana A, Kovanci E, Huszar G. Sperm maturity and treatment choice of in vitro fertilization (IVF) or intracytoplasmic sperm injection: diminished sperm HspA2 chaperone levels predict IVF failure. Fertil Steril. 2002;77(5):910–8.
Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, et al. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101(47):16501–6.
Li Y-F, He W, Mandal A, Kim Y-H, Digilio L, Klotz K, et al. CABYR binds to AKAP3 and Ropporin in the human sperm fibrous sheath. Asian J Androl. 2011;13(2):266.
Tanaka H, Iguchi N, Toyama Y, Kitamura K, Takahashi T, Kaseda K, et al. Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol. 2004;24(18):7958–64.
Siva AB, Kameshwari DB, Singh V, Pavani K, Sundaram CS, Rangaraj N, et al. Proteomics-based study on asthenozoospermia: differential expression of proteasome alpha complex. Mol Hum Reprod. 2010;16(7):452–62.
Liao T-T, Xiang Z, Zhu W-B, Fan L-Q. Proteome analysis of round-headed and normal spermatozoa by 2-D fluorescence difference gel electrophoresis and mass spectrometry. Asian J Androl. 2009;11(6):683–93.
Hosseinifar H, Gourabi H, Salekdeh GH, Alikhani M, Mirshahvaladi S, Sabbaghian M, et al. Study of sperm protein profile in men with and without varicocele using two-dimensional gel electrophoresis. Urology. 2013;81(2):293–300.
Cedenho A, Lima S, Cenedeze M, Spaine D, Ortiz V, Oehninger S. Oligozoospermia and heat-shock protein expression in ejaculated spermatozoa. Hum Reprod. 2006;21(7):1791–4.
Rapuling L. Proteomic analysis of human sperm proteins in relation to sperm motility, morphology and energy metabolism. Stellenbosch: University of Stellenbosch; 2010.
Amaral A, Castillo J, Estanyol JM, Ballescà JL, Ramalho-Santos J, Oliva R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol Cell Proteomics. 2013;12(2):330–42.
Martinez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballesca JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23(4):783–91. Epub 2008/02/19.
Zhao C, Huo R, Wang F-Q, Lin M, Zhou Z-M, Sha J-H. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil Steril. 2007;87(2):436–8.
Sarkar P, Collier TS, Randall SM, Muddiman DC, Rao BM. The subcellular proteome of undifferentiated human embryonic stem cells. Proteomics. 2012;12(3):421–30.
Organization WH. WHO laboratory manual for the examination and processing of human semen. 2010.
Baker MA, Naumovski N, Hetherington L, Weinberg A, Velkov T, Aitken RJ. Head and flagella subcompartmental proteomic analysis of human spermatozoa. Proteomics. 2013;13(1):61–74.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–54.
Hashemitabar M, Bahmanzadeh M, Mostafaie A, Orazizadeh M, Farimani M, Nikbakht R. A proteomic analysis of human follicular fluid: comparison between younger and older women with normal FSH levels. Int J Mol Sci. 2014;15(10):17518–40.
Mostafaie A, Yari K, Kiani S. A comparative evaluation of rehydration and cuploading sample application for modified twodimensional gel electrophoresis of human serum proteins using immobilized pH gradient. Afr J Biotechnol. 2013;10(55):11711–5.
Peknicova J, Pexidrova M, Kubatova A, Koubek P, Tepla O, Sulimenko T, et al. Expression of beta-tubulin epitope in human sperm with pathological spermiogram. Fertil Steril. 2007;88(4):1120–8.
Fackenthal JD, Turner FR, Raff EC. Tissue-specific microtubule functions in drosophila spermatogenesis require the β2-tubulin isotype-specific carboxy terminus. Dev Biol. 1993;158(1):213–27.
Chemes H, Olmedo SB, Carrere C, Oses R, Carizza C, Leisner M, et al. Ultrastructural pathology of the sperm flagellum: association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum Reprod. 1998;13(9):2521–6.
Cao W, Gerton GL, Moss SB. Proteomic profiling of accessory structures from the mouse sperm flagellum. Mol Cell Proteomics. 2006;5(5):801–10.
Brohmann H, Pinnecke S, Hoyer-Fender S. Identification and characterization of new cDNAs encoding outer dense fiber proteins of rat sperm. J Biol Chem. 1997;272(15):10327–32.
Petersen C, Füzesi L, Hoyer-Fender S. Outer dense fibre proteins from human sperm tail: molecular cloning and expression analyses of two cDNA transcripts encoding proteins of ~70 kDa. Mol Hum Reprod. 1999;5(7):627–35.
Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61(1):103–15.
Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol. 2002;248(2):331–42.
Baccetti B, Collodel G, Gambera L, Moretti E, Serafini F, Piomboni P. Fluorescence in situ hybridization and molecular studies in infertile men with dysplasia of the fibrous sheath. Fertil Steril. 2005;84(1):123–9.
Chan C-C, Shui H-A, Wu C-H, Wang C-Y, Sun G-H, Chen H-M, et al. Motility and protein phosphorylation in healthy and asthenozoospermic sperm. J Proteome Res. 2009;8(11):5382–6.
Yunes R, Doncel GF, Acosta AA. Incidence of sperm-tail tyrosine phosphorylation and hyperactivated motility in normozoospermic and asthenozoospermic human sperm samples. Biocell-Mendoza. 2003;27(1):29–36.
Ibrahim NM, Gilbert GR, Loseth KJ, Crabo BG. Correlation between clusterin‐positive spermatozoa determined by flow cytometry in bull semen and fertility. J Androl. 2000;21(6):887–94.
Francavilla S, Pelliccione F, Cordeschi G, Necozione S, Santucci R, Bocchio M, et al. Utrastructural analysis of asthenozoospermic ejaculates in the era of assisted procreation. Fertil Steril. 2006;85(4):940–6.
Suryawanshi AR, Khan SA, Gajbhiye RK, Gurav MY, Khole VV. Differential proteomics leads to identification of domain‐specific epididymal sperm proteins. J Androl. 2011;32(3):240–59.
Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285(5432):1393–6.
Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987;8(5):338–48.
Meseguer M, de los Santos MJ, Simón C, Pellicer A, Remohí J, Garrido N. Effect of sperm glutathione peroxidases 1 and 4 on embryo asymmetry and blastocyst quality in oocyte donation cycles. Fertil Steril. 2006;86(5):1376–85.
Liu B, Wang P, Wang Z, Jia Y, Niu X, Wang W, et al. Analysis and difference of voltage-dependent anion channel mRNA in ejaculated spermatozoa from normozoospermic fertile donors and infertile patients with idiopathic asthenozoospermia. J Assist Reprod Genet. 2010;27(12):719–24.
Kwon W-S, Park Y-J, Mohamed E-SA, Pang M-G. Voltage-dependent anion channels are a key factor of male fertility. Fertil Steril. 2013;99(2):354–61.
Neuer A, Spandorfer S, Giraldo P, Dieterle S, Rosenwaks Z, Witkin S. The role of heat shock proteins in reproduction. Hum Reprod Update. 2000;6(2):149–59.
Dix DJ, Garges JB, Hong RL. Inhibition of hsp70–1 and hsp70–3 expression disrupts preimplantation embryogenesis and heightens embryo sensitivity to arsenic. Mol Reprod Dev. 1998;51(4):373–80.
Schneider H-C, Berthold J, Bauer MF, Dietmeier K, Guiard B, Brunner M et al. Mitochondrial Hsp70/MIM44 complex facilitates protein import. 1994.
Aitken J, Buckingham D, Krausz C. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol Reprod Dev. 1994;39(3):268–79.
Bush LA, Herr JC, Wolkowicz M, Sherman NE, Shore A, Flickinger CJ. A novel asparaginase‐like protein is a sperm autoantigen in rats. Mol Reprod Dev. 2002;62(2):233–47.
Shen S, Wang J, Liang J, He D. Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J Urol. 2013;31(6):1395–401.
Paasch U, Heidenreich F, Pursche T, Kuhlisch E, Kettner K, Grunewald S, et al. Identification of increased amounts of eppin protein complex components in sperm cells of diabetic and obese individuals by difference gel electrophoresis. Mol Cell Proteomics. 2011;10(8):M110–007187.
Ramalho-Santos J, Amaral S, Oliveira PJ. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008;4(1):46–54.
Mangoli E, Talebi AR, Anvari M, Pourentezari M. Effects of experimentally-induced diabetes on sperm parameters and chromatin quality in mice. Iran J Reprod Med. 2013;11(1):53.
Acknowledgments
We thank all members of the infertility research and treatment center of Khuzestan and in Particular Mrs. Adham and Mrs. Ghalambaz for assistance in semen collection and analysis.
Conflicts of interest
The authors declare no conflict of interest.
Funding
This work is a part of the PhD thesis of Susan Sabbagh and was funded by Grant No. 90, from Cellular and Molecular Research Center (CMRC 90), Ahvaz Jundishapour University of Medical Sciences, Ahvaz, Iran.
Authors’ contributions
Mahmoud Hashemitabar designed the project and revised manuscript.
Susan Sabbagh carried out the experiments, writing of the manuscript.
Mahmoud Orazizadeh participated in its design.
Maryam Bahmanzadeh participated in analysis of data and writing of the manuscript.
Atta Ghadiri helped to draft the manuscript.
All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
The molecular mechanism that impairs sperm function is not fully understood. The current study evaluated the protein expression of sperm tails in asthenozoospermia patients and identified 14 proteins that had altered amounts. These are conserved proteins and probably play a critical role in sperm function. These proteins might be used as markers for the better diagnosis of sperm dysfunctions, targets for male contraceptive development, and to predict infertility and embryo quality.
Rights and permissions
About this article
Cite this article
Hashemitabar, M., Sabbagh, S., Orazizadeh, M. et al. A proteomic analysis on human sperm tail: comparison between normozoospermia and asthenozoospermia. J Assist Reprod Genet 32, 853–863 (2015). https://doi.org/10.1007/s10815-015-0465-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0465-7