Abstract
Purpose
To analyze the abundance and difference of voltage-dependent anion channel (VDAC) mRNA in ejaculated spermatozoa from normozoospermic fertile donors and infertile patients with idiopathic asthenozoospermia.
Methods
High motile and low motile spermatozoa were separated respectively from ejaculates of 36 donors and 40 patients using a discontinuous Percoll gradient centrifugation. Real-Time PCR was performed to detect mRNA abundance and difference of three VDAC subtypes between two groups with different sperm motility.
Results
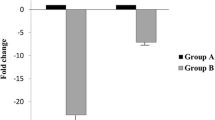
Real-Time PCR demonstrated that three VDAC mRNAs were present in mature spermatozoa. The VDAC2 mRNA level in ejaculated spermatozoa of patients was significantly higher than that of donors. No significant differences of VDAC1 and VDAC3 mRNA levels were found between two groups.
Conclusion
The high abundance of VDAC2 mRNA seemed to have a positive correlation with low sperm motility. The abnormal expression of VDAC might be related to male infertility with idiopathic asthenozoospermia.


Similar content being viewed by others
References
Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976;30:99–120.
Young MJ, Bay DC, Hausner G, Court DA. The evolutionary history of mitochondrial porins. BMC Evol Biol. 2007;7:31.
Decker WK, Bowles KR, Schatte EC, Towbin JA, Craigen WJ. Revised fine mapping of the human voltage-dependent anion channel loci by radiation hybrid analysis. Mamm Genome. 1999;10:1041–2.
Decker WK, Craigen WJ. The tissue-specific, alternatively spliced single ATG exon of the type 3 voltage-dependent anion channel gene does not create a truncated protein isoform in vivo. Mol Genet Metab. 2000;70:69–74.
Benz R. Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim Biophys Acta. 1994;1197:167–96.
Rostovtseva TK, Bezrukov SM. ATP transport through a single mitochondrial channel, VDAC, studied by current fluctuation analysis. Biophys J. 1998;74:2365–73.
Pavlov E, Grigoriev SM, Dejean LM, Zweihorn CL, Mannella CA, Kinnally KW. The mitochondrial channel VDAC has a cation-selective open state. Biochim Biophys Acta. 2005;1710:96–102.
Choudhary OP, Ujwal R, Kowallis W, Coalson R, Abramson J, Grabe M. The electrostatics of VDAC: implications for selectivity and gating. J Mol Biol. 2010;396:580–92.
Hodge T, Colombini M. Regulation of metabolite flux through voltage-gating of VDAC channels. J Membr Biol. 1997;157:271–9.
Tsujimoto Y, Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84:187–93.
Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–7.
Shoshan-Barmatz V, Hadad N, Feng W, Shafir I, Orr I, Varsanyi M, et al. VDAC/porin is present in sarcoplasmic reticulum from skeletal muscle. FEBS Lett. 1996;386:205–10.
Gonzalez-Gronow M, Kalfa T, Johnson CE, Gawdi G, Pizzo SV. The voltage-dependent anion channel is a receptor for plasminogen kringle 5 on human endothelial cells. J Biol Chem. 2003;278:27312–8.
Sabirov RZ, Sheiko T, Liu H, Deng D, Okada Y, Craigen WJ. Genetic demonstration that the plasma membrane maxianion channel and voltage-dependent anion channels are unrelated proteins. J Biol Chem. 2006;281:1897–904.
Hinsch KD, De Pinto V, Aires VA, Schneider X, Messina A, Hinsch E. Voltage-dependent anion-selective channels VDAC2 and VDAC3 are abundant proteins in bovine outer dense fibers, a cytoskeletal component of the sperm flagellum. J Biol Chem. 2004;279:15281–8.
Guarino F, Specchia V, Zapparoli G, Messina A, Aiello R, Bozzetti MP, et al. Expression and localization in spermatozoa of the mitochondrial porin isoform 2 in Drosophila melanogaster. Biochem Biophys Res Commun. 2006;346:665–70.
Arcelay E, Salicioni AM, Wertheimer E, Visconti PE. Identification of proteins undergoing tyrosine phosphorylation during mouse sperm capacitation. Int J Dev Biol. 2008;52:463–72.
Menzel VA, Cassará MC, Benz R, de Pinto V, Messina A, Cunsolo V, et al. Molecular and functional characterization of VDAC2 purified from mammal spermatozoa. Biosci Rep. 2009;29:351–62.
Liu B, Zhang W, Wang Z. Voltage-dependent anion channel in mammalian spermatozoa. Biochem Biophys Res Commun. 2010;397:633–6.
Sampson MJ, Decker WK, Beaudet AL, Ruitenbeek W, Armstrong D, Hicks MJ, et al. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J Biol Chem. 2001;276:39206–12.
Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, Sardi Segovia LM, et al. Asthenozoospermia: analysis of a large population. Arch Androl. 2003;49:343–9.
Liu B, Wang Z, Zhang W, Wang X. Expression and localization of voltage-dependent anion channels (VDAC) in human spermatozoa. Biochem Biophys Res Commun. 2009;378:366–70.
World Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. UK: Cambridge University Press; 1999.
Lambard S, Galeraud-Denis I, Bouraïma H, Bourguiba S, Chocat A, Carreau S. Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Mol Hum Reprod. 2003;9:117–24.
Lambard S, Galeraud-Denis I, Martin G, Levy R, Chocat A, Carreau S. Analysis and significance of mRNA in human ejaculated sperm from normozoospermic donors: relationship to sperm motility and capacitation. Mol Hum Reprod. 2004;10:535–41.
Lambard S, Galeraud-Denis I, Saunders PT, Carreau S. Human immature germ cells and ejaculated spermatozoa contain aromatase and oestrogen receptors. J Mol Endocrinol. 2004;32:279–89.
Ostermeier GC, Dix DJ, Miller D, Khatri P, Krawetz SA. Spermatozoal RNA profiles of normal fertile men. Lancet. 2002;360:772–7.
Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154.
Wang H, Zhou Z, Xu M, Li J, Xiao J, Xu ZY, et al. Spermatogenesis-related gene expression profile in human spermatozoa and its potential clinical applications. J Mol Med. 2004;82:317–24.
García-Herrero S, Garrido N, Martínez-Conejero JA, Remohí J, Pellicer A, Meseguer M. Ontological evaluation of transcriptional differences between sperm of infertile males and fertile donors using microarray analysis. J Assist Reprod Genet. 2010;27:111–20.
Messina A, Oliva M, Rosato C, Huizing M, Ruitenbeek W, van den Heuvel LP, et al. Mapping of the human voltage-dependent anion channel isoforms 1 and 2 reconsidered. Biochem Biophys Res Commun. 1999;255:707–10.
Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med. 2010;31:227–85.
Hinsch KD, Asmarinah, Hinsch E, Konrad L. VDAC2 (porin-2) expression pattern and localization in the bovine testis. Biochim Biophys Acta. 2001;1518:329–33.
Flörke H, Thinnes FP, Winkelbach H, Stadtmüller U, Paetzold G, Morys-Wortmann C, et al. Channel active mammalian porin, purified from crude membrane fractions of human B lymphocytes and bovine skeletal muscle, reversibly binds adenosine triphosphate (ATP). Biol Chem Hoppe Seyler. 1994;375:513–20.
Triphan X, Menzel VA, Petrunkina AM, Cassará MC, Wemheuer W, Hinsch KD, et al. Localisation and function of voltage-dependent anion channels (VDAC) in bovine spermatozoa. Pflugers Arch. 2008;455:677–86.
Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod. 2003;68:1590–6.
Luconi M, Porazzi I, Ferruzzi P, Marchiani S, Forti G, Baldi E. Tyrosine phosphorylation of the A kinase anchoring protein 3 (AKAP3) and soluble adenylate cyclase are involved in the increase of human sperm motility by bicarbonate. Biol Reprod. 2005;72:22–32.
Krasznai Z, Krasznai ZT, Morisawa M, Bazsáné ZK, Hernádi Z, Fazekas Z, et al. Role of the Na+/Ca2+ exchanger in calcium homeostasis and human sperm motility regulation. Cell Motil Cytoskeleton. 2006;63:66–76.
Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, et al. Glyceraldehyde 3-phosphate dehydrogenase-S, a spermspecific glycolytic enzyme, is required for sperm motility and male fertility. Proc NatlAcad Sci USA. 2004;101:16501–6.
Acknowledgments
We thank Key Laboratory of Reproductive Medicine, Institute of Toxicology, Nanjing Medical University for the technical assistance. This work was supported by the grant from National Natural Science Foundation of China (30872575).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Capsule
The high abundance of VDAC2 mRNA seemed to have a positive correlation with low sperm motility in male infertility with idiopathic asthenozoospermia.
Rights and permissions
About this article
Cite this article
Liu, B., Wang, P., Wang, Z. et al. Analysis and difference of voltage-dependent anion channel mRNA in ejaculated spermatozoa from normozoospermic fertile donors and infertile patients with idiopathic asthenozoospermia. J Assist Reprod Genet 27, 719–724 (2010). https://doi.org/10.1007/s10815-010-9466-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-010-9466-8