Abstract
Phycobiliproteins are pigments with uses in pharmacology, cosmetics, foods, and as fluorescent probes in biochemistry. Cryptophyte microalgae are one possible source of phycobiliproteins as well as other molecules such as omega-3 fatty acids. The use of cryptophytes in biotechnology is currently very limited and especially the potential of freshwater species is poorly documented. For commercial microalgae production, it is important to find the best performing strains in terms of growth and yields of the products of interest. Phycoerythrin is a phycobiliprotein with red colour and strong yellow fluorescence. In this study, we evaluate the growth and phycoerythrin production of eight strains of freshwater cryptophytes belonging to the genus Cryptomonas, comparing them to two marine strains. The strains are grown in batch cultures under standardised conditions. Most of the studied freshwater strains have lower growth rates and all of them have lower biomass yields than the marine strains. However, most of them have much higher cellular phycoerythrin concentrations, which in the case of two strains leads them to a significantly higher overall phycoerythrin yield. There is large variation among cryptophytes in growth rates and phycoerythrin content. Our results suggest that freshwater cryptophytes of the genus Cryptomonas may be better sources of phycoerythrin than the more extensively studied marine strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phycobiliproteins (PBPs) are a group of proteinaceous pigments which form part of the light harvesting structures in red algae, cyanobacteria and cryptophyte microalgae. They are accessory pigments that allow these organisms to harvest light energy outside the wavelengths absorbed by chlorophyll and carotenoids. PBPs can be divided into groups based on their wavelength of maximum absorption, phycoerythrins (PEs) having a peak at 545–566 nm, phycoerythrocyanins at 480–580 nm, phycocyanins at 569–645 nm and allophycocyanins at 540–671 nm (Glazer and Bryant 1975; MacColl and Guard-Friar 1987; Hill and Rowan 1989; Stadnichuk et al. 2015). PBPs are used for applications in pharmacology due to their antioxidative, anti-tumour and photosensitizing properties as well as their usefulness as fluorescent markers (Li et al. 2019). They are also being studied for potential applications in photovoltaic devices (Frias et al. 2019). PE is used as colourant in cosmetics, while phycocyanin sees use in both foods and cosmetics (Sekar and Chandramohan 2008).
Phycocyanin is produced commercially using strains of cyanobacteria such as Arthrospira platensis (Eriksen 2008). Promising organisms for PE production include the genus Porphyridium (Kathiresan et al. 2007) as well as certain species of red macroalgae (Rhodophyta). Use of phycocyanin is currently more extensive than that of PE and it is approved for use as food colourant in many markets. While commercial production of PBPs by means of microalgal cultivation is already practised, decreases in the production cost would enable the broader use of PBPs in dyes and colourants, potentially replacing products of petrochemical and agricultural origins that have higher environmental impacts.
Cryptophytes are a taxon of microalgae found in most aquatic habitats in which they are often important primary producers. Cryptophytes produce several kinds of secondary metabolites that could see wide use in industry, but research on them is still scarce and most of their potential in biotechnology remains unexplored (Abidizadegan et al. 2021). Between 12 and 22% of the total protein in cryptophyte cells can consist of PBPs (da Silva et al. 2009; Seixas et al. 2009).
Additionally, cryptophytes are known to produce omega-3 polyunsaturated fatty acids (ω-3 PUFAs) (Taipale et al. 2013) and exopolysaccharides (Giroldo and Vieira 2002). They are widely studied in ecology but there is a lack of knowledge on their use for applied purposes. Especially growth rates, yields of bioactive compounds and optimal growing conditions are known for few strains. Cryptophyte genera belonging to the order Pyrenomonadales, such as Rhodomonas, Teleaulax and Proteomonas have been previously suggested for use in biotechnology (Lee et al. 2019) and are known to be good producers of the ω-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Peltomaa et al. 2018). These species are mostly marine. Species of the exclusively freshwater genus Cryptomonas that belong to the distinct order Cryptomonadales produce EPA and DHA similarly to the above-mentioned genera (Peltomaa et al. 2017).
Cultivation of marine strains of microalgae is often seen as advantageous since this does not consume freshwater resources that could be used for drinking water purposes, therefore reducing the water footprint of the end-products (Maeda et al. 2018). However, the cultivation of microalgae should be adapted to the local conditions and there are many areas of the world where freshwater is plentiful and saltwater scarce. Furthermore, the costs of growing marine algae far from the sea are increased by the need to treat the salty effluents before their release into the environment.
In biotechnology cryptophytes would have several advantages compared to other PBP producing organisms. Firstly, each strain produces only one type of PBP (Glazer and Wedemayer 1995) in contrast to the phycobilisome protein complexes produced by red algae and cyanobacteria, which always contain allophycocyanin and phycocyanin and depending on the species, also PE or phycoerythrocyanin (Stadnichuk et al. 2015). This facilitates a simpler protein purification in the production process of PBPs, since it eliminates the need to separate different PBPs from each other. Additionally, the low molecular weight of cryptophycean PBPs compared to PBPs of cyanobacterial or red algal origin may make them more useful in fluorescent labelling (Telford et al. 2001). Secondly, cryptophytes also produce both EPA and DHA (Peltomaa et al. 2018), while most algae produce at most one of them in significant quantities. These two ω-3 PUFAs are produced mainly by aquatic primary producers but their presence in the diet is important for the health of humans and many animal species (Hixson et al. 2015), and thus, ω-3 PUFAs are also used in aquaculture feeds. Currently, their main sources in human diets are fish-based products. Thirdly, cryptophytes lack a strong cell wall, which simplifies biomass processing, eliminating the need for cell wall disruption steps in the extraction of PBPs and other intracellular compounds. The lack of heavy protective structures also means that a higher proportion of the total biomass may consist of biologically active components.
The commercialization of products derived from microalgae is limited due to the high production costs (Medeiros et al. 2021). Besides technical innovations, these costs can be reduced by identifying wild strains or creating genetically engineered strains that grow rapidly and contain high concentrations of the desired products. Another strategy for cost reduction is the so-called biorefinery concept where the biomass is processed into different fractions to extract as much value as possible (Zhu 2015). This requires strains that simultaneously produce several desirable products. PBPs are water-soluble and therefore their extraction does not require the use of organic solvents (cf. conventional extraction of carotenoids). The simultaneous production of PBPs and ω-3 PUFAs in a biorefinery concept is especially interesting since processing the biomass into PBP-rich hydrophilic and PUFA-rich hydrophobic fractions would be relatively easy. Separation would be more laborious if producing two hydrophobic fractions instead, such as carotenoids and PUFAs.
Cryptophytes could be suitable for production of PBPs in a biorefinery concept due to their known benefits over other types of algae. However, it is known that growth rates vary between different cryptophyte strains e.g. (Peltomaa et al. 2018). The amount of PBPs in cells of cryptophytes also varies depending on the strain (Cunningham et al. 2019) as well as the abiotic conditions such as temperature (Chaloub et al. 2015), available light spectrum (Heidenreich and Richardson 2020), light intensity and nitrogen availability (Lewitus and Caron 1990).
The present study aims to identify suitable cryptophytes for PE production among ten strains, grown in standardised conditions. We used strains with the information that they can be reliably grown in laboratory conditions. We compared the growth rates, dry weight and PBP productivities of eight freshwater cryptophytes from the genus Cryptomonas and two marine strains from the genera Rhodomonas and Proteomonas. We cultured the algae in controlled conditions in a batch experiment and followed their growth. Besides the amount of PBPs, we also measured dry weight of the produced biomass. The aim was to identify the strains, which produce high yields of biomass containing high concentrations of PE. Promising strains can then be studied further to identify their optimal growing conditions.
Materials and methods
Strains and culturing
All strains were acquired from culture collections. The studied strains along with their culture collection of origin and their habitat are shown in Table 1.
Freshwater strains were grown in MWC medium (Guillard and Lorenzen 1972) and marine strains in f/2 medium (Guillard and Ryther 1962) prepared using artificial sea salt (Dupla Marin Natural Balance, Dohse Aquaristik GmbH & Co. KG, Germany) (see Online Resource 1 for exact media compositions). There is evidence that PBPs may function as a cellular nitrogen store and that under nitrogen starvation, algal cells use nitrogen from PBPs to sustain cell metabolism causing a decrease in cellular PBP content (Bartual et al. 2002). The growth media utilised was thus chosen to have sufficient nitrogen content. The stock cultures were grown under the same conditions as the experimental cultures. For the growth experiment, the algae from the exponential growth phase were inoculated into 600 mL plastic cell culture flasks with fresh medium. The culture volume was 445 mL made up of 45 mL of inoculum and 400 mL of fresh medium (cf. Sánchez-Bayo et al. 2020). The experiment was performed in triplicate, and all culture vessels were sampled to obtain the average growth rates. The algae were grown in a growth cabinet (Friocell Evo 404, MMM Group, Germany) at 20 °C under constant illumination by white LED-lights and a photosynthetic photon flux density (PPFD) of 200 µmol photons m−2 s−1, measured with a LI-190 Quantum Sensor (LI-COR Biosciences UK Ltd, UK). The cultures were mixed daily by gently shaking them manually before rearranging the bottles in a random order.
Cell concentrations, dry weight and growth rates
Cell densities were determined regularly to follow the growth of cultures. Samples of 1.5 mL were collected into plastic microcentrifuge tubes. A drop of Lugol’s solution (Willén 1962) was added to each tube for preservation and the tubes were stored at 4 °C until cells were counted with a Sedgewick Rafter counting chamber using an Olympus CX40 microscope and 100 × total magnification. A minimum of 100 cells or 20 squares were counted for each sample (Lund et al. 1958).
Specific growth rates (day−1) were calculated based on the cell densities. A least-squares fit of the straight line to logarithmically transformed data was used as described by Guillard (1973). The R code for this is available in Online Resource 2. Only fits with a R2 value of 0.8 or higher were accepted. Cell densities from at least three separate samplings were used for the calculations.
For freshwater strains, the experiment was ended once the cultures reached their maximum density and for the marine strains on the tenth day of culturing. The cultures were then sampled for dry weight. Glass fibre filters (Whatman GF/C, ∅ 47 mm) were dried at 105 °C overnight and weighted. Using a vacuum filtration apparatus, 10–100 mL of culture was filtered, and the filters were dried overnight at 105 °C and weighted again. Immediately after sampling for dry weight determination, samples were drawn for PBP extraction (see below).
Phycobiliproteins
PBP extraction was done similarly to the method proven effective in the comparison by Lawrenz et al. (2011) (treatment c). For the extraction, at the end of the experiment, 10 mL of well-mixed culture were centrifuged at 2000 × g for 10 min (Heraeus Multifuge 1 S-R, Kendro Laboratory Products, Germany) and the supernatant replaced with 8 mL of 0.1 M phosphate buffer. The tubes were then kept overnight in a freezer at –20 °C with the purpose of rupturing the cells and releasing the PBPs. The samples were extracted at 5 °C for 24 h. For removing cell debris, the samples were centrifuged at 3500 × g for 15 min and the supernatant collected into a clean tube.
PBPs were identified optically, by measuring the absorption spectra of the extracts. A Shimadzu UV-2401PC spectrophotometer (Shimadzu Corporation, Japan) was used with two cm long glass cuvettes. Phosphate buffer was used as the blank sample. The spectra were recorded from 280 to 800 nm at 1 nm intervals. Scatter correction was done by subtracting the absorbance at 750 nm from the peak absorbance of the PBP (as listed in Table 2) (Lawrenz et al. 2011).
The PBP concentrations (mg L−1) were calculated according to Eq. 1 adapted from the article of Cunningham et al. (2019), where A = absorbance of sample at 565 nm for freshwater strains or at 548 nm for marine strains, ε = the extinction coefficient for cryptophycean PE (5.67 × 105 L mol−1 cm−1), MW = molecular weight of cryptophycean PE (45 000 Da), d = path length of the cuvette in cm, Vbuffer and Vsample = volume of buffer and sample, respectively, and 103 is a conversion factor to convert the result to mg L−1. The extinction coefficient and molecular weights used by Cunningham et al. (2019) were chosen because they have been determined for cryptophytes, not for cyanobacteria or red algae, which have PBPs of differing molecular weights (Becker et al. 1998).
Statistical analysis
The data were tested for normality using Levene’s test with a p-value limit of 0.05 and found to be normally distributed. Therefore, one-way analysis of variance (ANOVA) was used to test for differences between strains together with Tukey’s honestly significant difference test (Tukey’s HSD) for pairwise differences. The analysis was done in R version 4.0.3 (R Core Team 2020) and the plots were produced using the ggplot2 package (Wickham 2016). All data and R code used to process them are provided in Online Resource 2. The full results are provided in Online resource 5.
Results
Growth and biomass yield
Dry weight at the end of the growth experiments varied between 0.016 (C. erosa) and 0.124 g L−1 (C. ozolinii) for the freshwater strains; the difference between these two strains was the only statistically significant difference among the freshwater strains (ANOVA: F9,20 = 15.84, p < 0.001; Tukey’s HSD: p < 0.05). The marine strains P. sulcata and R. salina reached a higher dry weight of 0.229 g L−1 and 0.235 g L−1, respectively, and differed from the freshwater strains (Fig. 1).
Specific growth rates of the strains varied from 0.122 to 0.322 day−1. C. pyrenoidifera NIVA 2/81 and C. ozolinii had higher growth rates than the other freshwater strains (Table 2). The growth rate for C. erosa could not be determined due to poor fit of the data (R2 < 0.8). No significant differences between freshwater and marine strains were found, except for C. sp CPCC 336, which had a lower growth rate than the marine strains (ANOVA: F9,20 = 10.47, p < 0.001; Tukey’s HSD: p < 0.05). Growth curves based on cell densities are shown in Online Resource 3.
Phycobiliproteins
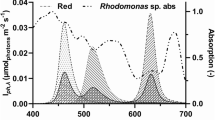
All freshwater strains produced PE with the main absorption peak at 565 nm while both marine strains had the peak at 548 nm (Table 2). The wide spectral scans showed only a single major peak above 280 nm, confirming that the extraction was successful. Sample spectra are shown in Online Resource 4.
Cryptomonas curvata, C. erosa, C. lundii, C marssonii and C. pyrenoidifera CCAP 979/61 had higher concentrations of PE per cell than the marine strains (ANOVA: F9,20 = 17.16, p < 0.001; Tukey’s HSD: p < 0.05) (Table 2). The concentration in freshwater strains ranged between 3.0 and 73 pg cell−1, while the marine R. salina and P. sulcata had concentrations of 2.8 and 3.2 pg cell−1, respectively.
The highest PE concentrations per volume of culture were produced by C. curvata (0.71 mg L−1). Other freshwater strains producing higher concentrations than marine strains were C. lundii (0.45 mg L−1) and C. marssonii (0.31 mg L−1) (ANOVA: F9,20 = 92.64, p < 0.001; Tukey’s HSD: p < 0.05) (Table 2). The concentrations in freshwater strains ranged from 0.065 to 0.71, while the marine R. salina and P. sulcata had concentrations of 0.15 and 0.19 mg L−1, respectively.
The highest concentration of PE in biomass was 7.9 mg g−1, produced by C. curvata (Table 2). Cryptomonas erosa, C. lundii, C. marssonii and C. pyrenoidifera CCAP 979/61 produced concentrations between 4.0 and 5.4 mg g−1, i.e. above the concentrations produced by the marine strains P. sulcata (0.92 mg g−1) and R. salina (0.70 mg g−1) (ANOVA: F9,20 = 77.64, P < 0.001; Tukey’s HSD: p < 0.05).
Highest daily yields per units of volume were given by C. curvata (0.047 mg L−1 day−1) followed by C. lundii (0.030 mg L−1 day−1) (Fig. 2). C. marssonii and C. pyrenoidifera CCAP 979/61 produced yields comparable to the marine strains P. sulcata and R. salina, which had yields of 0.015 and 0.019 mg L−1 day−1, respectively (ANOVA: F9,20 = 80.88, p < 0.001; Tukey’s HSD: p < 0.05).
Discussion
Dry weights in marine strains were approximately twice as high as in freshwater strains. Among the freshwater strains, none stood out as clearly superior in terms of dry weight. For two strains of Rhodomonas salina, dry weights between 0.25 and 1.05 g L−1 were reported under different nutrient concentrations, temperatures and PPFDs (Guevara et al. 2016). However, the above values for cryptophytes are low compared to those reported for other microalgae. Up to 20 g L−1 and 2.11 g L−1 day−1 has been reported for optimised cultures of Chlorella vulgaris, for instance (Fu et al. 2012). Peltomaa et al. (2018) reported daily dry weight yields of 3.4, 2.79 and 1.37 mg L−1 day−1 for the marine cryptophytes Chroomonas mesostigmatica, Rhodomonas salina and Proteomonas sulcata.
Specific growth rates for freshwater strains in this study were between 0.122 and 0.322 day−1 and for marine strains between 0.226 and 0.232 day−1. No clear distinction between freshwater and marine strains could be made. Growth rates were lower compared to those reported for marine strains elsewhere. A growth rate of 1.2 day−1 was observed in a marine cryptophyte Pyrenomonas salina at PPFDs of 115 µmol photons m−2 s−1 and above (Lewitus and Caron 1990), and 0.68 day−1 in a marine Rhodomonas sp. at 50 µmol photons m−2 s−1 (da Silva et al. 2009). However, the cellular PE concentrations in these two studies reached only 5 pg cell−1. Peltomaa et al. (2018) reported growth rates between 0.34 and 0.70 day−1 for marine cryptophytes. For freshwater Cryptomonas marssonii, a maximum growth rate of 0.56 day−1 was reported at 11 °C and 0.50 day−1 at 20 °C (Butterwick et al. 2005). Specific growth rates of 0.67 and 1.39 day−1 were reported for two freshwater Cryptomonas strains (Ojala 1993). Optimal growth in two freshwater Cryptomonas strains was found to be at 19 and 24.5 °C (Ojala 1993) and according to Cloern (1977), the temperature optimum of Cryptomonas ovata is between 20 and 26 °C. According to Butterwick et al. (2005), the highest growth rate for Cryptomonas marssonii was at 11 °C, but only a small decline was found from 11 to 20 °C. It is therefore probable that the temperature chosen for our experiments was close to the optimum although some strains might have benefited from a lower temperature. Somewhat higher dry weights and growth rates compared to our results have been reported elsewhere, especially in studies of marine cryptophyte species. However, the aim of this study was not to optimise growing conditions, but to compare a range of different strains.
All freshwater strains selected in this study belong to the genus Cryptomonas. They produced PE with the maximum absorption at 565 nm, while the marine strains produced PE with the maximum absorption at 548 nm, both confirming previous reports (cf. Hill and Rowan 1989). PE yield did not follow the pattern of dry weight production. While the highest dry weights were produced by the two marine strains, their cellular PE concentrations were among the lowest of the tested strains. The biochemical composition of microalgae can change according to growth phase. In the marine cryptophyte P. salina, the cellular concentration of PE strongly decreased in the stationary phase when the culture was nitrogen or phosphorus depleted (Lewitus and Caron 1990). Likewise, in Rhodomonas sp. PE was depleted under nitrogen starvation (da Silva et al. 2009). The low cellular PE concentrations in our marine strains compared to our freshwater strains are not explained by differences in nitrogen starting concentrations, since nutrient medium for freshwater strains had 1 mmol L−1 of nitrate and for marine strains 0.88 mmol L−1 (see Online Supplementary Material 1). The duration of stationary phase before harvest could explain some of the differences in PE content among the freshwater strains, but the difference compared to marine strains is more likely to be genetic, since the marine strains hardly reached the stationary phase, yet had the lowest PE contents per cell.
The light intensity used in this experiment was in the high range of what is typically used when culturing cryptophytes. The high cellular PE concentrations reached compared to other studies, would hint towards a photoprotective response, however no photoprotective function for PBPs has been described. On the opposite, PBP concentrations have been shown to decline under increasing light intensities at least in a marine Rhodomonas strain (Chaloub et al. 2015). While cryptophytes are known to thrive under low PPFDs, Oostlander et al. (2020) found that, in a marine Rhodomonas sp., dry weight increased at least until 600 µmol photons m−2 s−1 and biomass yield at least up to a PPFD of 330 µmol photons m−2 s−1. In two strains of the marine R. salina, higher growth rates and maximum cell densities were found under 200 µmol photons m−2 s−1 compared to 100 µmol photons m−2 s−1 (Guevara et al. 2016). For two freshwater Cryptomonas species grown at 21 °C, Ojala (1993) showed that, the highest growth rates were found at a PPFD close to 200 µmol photons m−2 s−1, and growth was declining only slowly at higher PPFDs.
Heidenreich and Richardson (2020) measured PE concentrations of 6 and 8 pg cell−1 for P. sulcata and R. salina (under full spectrum light), while we measured concentrations of 3.2 and 2.8 pg cell−1. Cunningham et al. (2019) reported the following PBP concentrations for freshwater strains: C. ovata, 26 pg cell−1; C. pyrenoidifera, 40 pg cell−1; Cryptomonas sp., 16 pg cell−1; C. curvata, 17 pg cell−1; and for marine strains P. sulcata, 10 pg cell−1; R. salina 11 pg cell−1. The studies used PPFDs of 32 and 30 µmol photons s−1 m−2, while we used 200 µmol photons s−1 m−2. While the results are not entirely in agreement with ours, they support our observation that freshwater Cryptomonas species have higher cellular PBP concentrations compared to marine species of cryptophytes.
The most rapidly dividing strains can be expected to have lower PBP concentrations as they are using their metabolic capacity for essential cell components. Because PEs are proteins, their synthesis can compete with that of other proteins. It is probable that the lower PE synthesis in marine cryptophyte strains is a factor that enables them to divide more rapidly. Thus, it also makes sense that our freshwater strains, while having lower growth, had higher PBP concentrations. In algal culturing, the best yields are sometimes reached with strains that do not produce the highest concentrations but grow rapidly (Griffiths and Harrison 2009).
Latsos et al. (2021) reported a much higher PE concentration of 23 mg g−1 of dry weight than measured in our experiment for a marine Rhodomonas sp. However, because of the large differences in cellular PE concentrations, the volumetric yields (in mg L−1) of PE do not correspond to the dry weight yields. Instead of R. salina and P. sulcata, the highest volumetric PE yields were recorded in the freshwater strains C. curvata and C. lundii. Because of the differing growth rates and cell densities, the volumetric PE yields do not follow the cellular concentrations of PE either. For example, C. erosa had one of the highest concentrations of PE per cell but very low overall yields. PE concentrations in biomass followed a somewhat similar pattern to the volumetric yield where C. curvata had the highest concentrations.
When R. salina was grown under green light, a biomass yield of 0.2 g L−1 day−1 together with a PE concentration of 114 mg g−1 DW were measured (Latsos et al. 2021). This would give a PE yield of 22.8 mg L−1 day−1. In an optimised culture of Porphyridium purpureum, a dry weight yield of 9.12 g L−1 containing 3,09% w/w of PBPs and 2.20% w/w of PE was reported (Kathiresan et al. 2007), which would correspond to a yield of 200 mg L−1 of PE. A yield of 33.3 mg L−1 of total PBPs was obtained in a different experiment using the same species (Guihéneuf and Stengel 2015). However, as noted in the introduction, the phycoerythrin produced by cryptophytes differs from phycoerythrins of other algae.
Conclusions
The yield of a specific compound from microalgal cultivation is the result of an interaction between the concentration of that compound in the harvested biomass and the yields of that biomass per unit of time and of culture volume. This is best represented by the yield in mg L−1 day−1 of the compound in question.
Our results show that the PE yield of a strain cannot be predicted solely on the dry weight production, nor the PE concentration per cell. The two marine strains did not produce the highest yields of PE despite producing the highest biomass yields. This is due to the lower concentrations of PE in the cells of these strains and consequently in the biomass.
The overall biomass and PE productivities reported in this study are relatively low compared to values reported elsewhere (see above). However, the growth conditions were purposefully kept simple in order to be able to compare many strains. Now that the most promising strains have been identified, and with the addition of e.g. proper mixing, CO2 addition and optimized light availability, the growth can be expected to improve.
Freshwater and marine strains were grown under the same conditions. When comparing the highest yielding freshwater strain (C. curvata) to the highest yielding marine strain (P. sulcata), our results show up to 22 times higher cellular PE concentrations, up to 3.7 times higher concentrations per culture volume, up to 8.6 times higher concentrations in biomass and up to 2.5 times higher daily volumetric yields in C. curvata.
Commercial microalgal cultivation usually aims at a continuous culture where the algae are kept in the phase of exponential growth. Batch culture experiments such as this one can only give a limited view of the possible productivity of such a system. The differences between strains in specific growth rate and PE productivity together with the large variation of PE concentration in cells (a more than 25-fold difference between the lowest and highest strain) show that careful consideration is needed when selecting strains for large-scale PE production. For developing the biorefinery concept, the best performing strains should also be compared for their yields of EPA and DHA.
The highest yields of PE in mg L−1 and mg L−1 day−1 were recorded in C. curvata followed by C. lundii. Therefore, we consider these two strains as the most promising for further experiments to determine optimal growth conditions for maximal PE yields in cryptophytes.
Availability of data and material
All data generated or analysed during this study are included in this published article and its online supplementary material.
Code availability
All custom code created during this study is included in the online supplementary material.
References
Abidizadegan M, Peltomaa F, Blomster J (2021) The potential of cryptophyte algae in biomedical and pharmaceutical applications. Front Pharmacol 11:17
Bartual A, Lubián LM, Gálvez JA, Niell FX (2002) Effect of irradiance on growth, photosynthesis, pigment content and nutrient consumption in dense cultures of Rhodomonas salina (Wislouch) (Cryptophyceae). Cienc Mar 28:381–392
Becker M, Stubbs MT, Huber R (1998) Crystallization of phycoerythrin 545 of Rhodomonas lens using detergents and unusual additives. Protein Sci 7:580–586
Butterwick C, Heaney SI, Talling F (2005) Diversity in the influence of temperature on the growth rates of freshwater algae, and its ecological relevance. Freshw Biol 50:291–300
Chaloub RM, Motta NMS, de Araujo SP, de Aguiar de PF, da Silva AF, (2015) Combined effects of irradiance, temperature and nitrate concentration on phycoerythrin content in the microalga Rhodomonas sp. (Cryptophyceae). Algal Res 8:89–94
Cloern JE (1977) Effects of light intensity and temperature on Cryptomonas ovata (Cryptophyceae) growth and nutrient uptake rates. J Phycol 13:389–395
Cunningham BR, Greenwold MJ, Lachenmyer EM, Heidenreich KM, Davis AC, Dudycha JL, Richardson TL (2019) Light capture and pigment diversity in marine and freshwater cryptophytes. J Phycol 55:552–564
da Silva AF, Lourenço SO, Chaloub RM (2009) Effects of nitrogen starvation on the photosynthetic physiology of a tropical marine microalga Rhodomonas sp. (Cryptophyceae). Aquat Bot 91:291–297
Eriksen NT (2008) Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14
Frias AR, Correia SFH, Martins M, Ventura SPM, Pecoraro E, Ribeiro SJL, André PS, Ferreira RAS, Coutinho JAP, Carlos LD (2019) Sustainable liquid luminescent solar concentrators Adv Sustain Syst 3:1800134
Fu W, Gudmundsson O, Feist AM, Herjolfsson G, Brynjolfsson S, Palsson BØ (2012) Maximizing biomass productivity and cell density of Chlorella vulgaris by using light-emitting diode-based photobioreactor. J Biotechnol 161:242–249
Giroldo D, Vieira AAH (2002) An extracellular sulfated fucose-rich polysaccharide produced by a tropical strain of Cryptomonas obovata (Cryptophyceae). J Appl Phycol 14:185–191
Glazer AN, Bryant DA (1975) Allophycocyanin B (λmax 671, 618 nm): A new cyanobacterial phycobiliprotein. Arch Microbiol 104:15–22
Glazer AN, Wedemayer GJ (1995) Cryptomonad biliproteins — an evolutionary perspective. Photosynth Res 46:93–105
MJ Griffiths STL Harrison (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guevara M, Arredondo-Vega BO, Palacios Y, Saéz K, Gómez PI (2016) Comparison of growth and biochemical parameters of two strains of Rhodomonas salina (Cryptophyceae) cultivated under different combinations of irradiance, temperature, and nutrients. J Appl Phycol 28:2651–2660
Guihéneuf F, Stengel DB (2015) Towards the biorefinery concept: interaction of light, temperature and nitrogen for optimizing the co-production of high-value compounds in Porphyridium purpureum. Algal Res 10:152–163
Guillard RRL (1973) Division rates. In: JR Stein (eds) Handbook of Phycological Methods: Culture Methods and Growth Measurements. Cambridge University Press, Cambridge, pp 289–311
Guillard RRL, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide c. J Phycol 8:10–14
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Heidenreich KM, Richardson TL (2020) Photopigment, absorption, and growth responses of marine cryptophytes to varying spectral irradiance. J Phycol 56:507–520
Hill DRA, Rowan KS (1989) The biliproteins of the Cryptophyceae. Phycologia 28:455–463
Hixson SM, Sharma B, Kainz MJ, Wacker A, Arts MT (2015) Production, distribution, and abundance of long-chain omega-3 polyunsaturated fatty acids: a fundamental dichotomy between freshwater and terrestrial ecosystems. Environ Rev 23:414–424
Kathiresan S, Sarada R, Bhattacharya S, Ravishankar GA (2007) Culture media optimization for growth and phycoerythrin production from Porphyridium purpureum. Biotechnol Bioeng 96:456–463
Latsos C, Houcke J, van L Blommaert GP Verbeeke J Kromkamp KR Timmermans, (2021) Effect of light quality and quantity on productivity and phycoerythrin concentration in the cryptophyte Rhodomonas sp. J Appl Phycol 33:1–13
Lawrenz E, Fedewa EJ, Richardson TL (2011) Extraction protocols for the quantification of phycobilins in aqueous phytoplankton extracts. J Appl Phycol 23:865–871
Lee BI, Kim SK, Kim JH, Kim HS, Kim JI, Shin W, Rho J-R, Yih W (2019) Intraspecific variations in macronutrient, amino acid, and fatty acid composition of mass-cultured Teleaulax amphioxeia (Cryptophyceae) strains. Algae 34:163–175
Lewitus AJ, Caron DA (1990) Relative effects of nitrogen or phosphorus depletion and light intensity on the pigmentation, chemical composition, and volume of Pyrenomonas salina (Cryptophyceae). Mar Ecol Prog Ser 61:171–181
Li W, Su H-N, Pu Y, Chen J, Liu L-N, Liu Q, Qin S (2019) Phycobiliproteins: molecular structure, production, applications, and prospects. Biotechnol Adv 37:340–353
Lund JWG, Kipling C, Le Cren ED (1958) The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11:143–170
MacColl R, Guard-Friar D (1987) Phycobiliproteins. CRC Press, Boca Raton, pp 45–67
Maeda Y, Yoshino T, Matsunaga T, Matsumoto M, Tanaka T (2018) Marine microalgae for production of biofuels and chemicals. Curr Opin Biotechnol 50:111–120
Medeiros VPB, de Costa WKA, da Silva RT, da Pimentel TC, Magnani M (2021) Microalgae as source of functional ingredients in new-generation foods: challenges, technological effects, biological activity, and regulatory issues. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1879729
Ojala A (1993) Effects of temperature and irradiance on the growth of two freshwater photosynthetic cryptophytes. J Phycol 29:278–284
Oostlander PC, van Houcke J, Wijffels RH, Barbosa MJ (2020) Optimization of Rhodomonas sp. under continuous cultivation for industrial applications in aquaculture. Algal Res 47:101889
Peltomaa E, Johnson MD, Taipale SJ (2018) Marine cryptophytes are great sources of EPA and DHA. Mar Drugs 16:3
Peltomaa ET, Aalto SL, Vuorio KM, Taipale SJ (2017) The importance of phytoplankton biomolecule availability for secondary production. Front Ecol Evol 5:128
R Core Team (2020) R: a language and environment for statistical computing Vienna, Austria
Sánchez-Bayo A, Morales V, Rodríguez R, Vicente G, Bautista LF (2020) Cultivation of microalgae and Cyanobacteria: effect of operating conditions on growth and biomass composition. Molecules 25:2834
Seixas P, Coutinho P, Ferreira M, Otero A (2009) Nutritional value of the cryptophyte Rhodomonas lens for Artemia sp. J Exp Mar Biol Ecol 381:1–9
Sekar S, Chandramohan M (2008) Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol 20:113–136
Stadnichuk IN, Krasilnikov PM, Zlenko DV (2015) Cyanobacterial phycobilisomes and phycobiliproteins. Microbiology 84:101–111
Taipale S, Strandberg U, Peltomaa E, Galloway A, Ojala A, Brett M (2013) Fatty acid composition as biomarkers of freshwater microalgae: analysis of 37 strains of microalgae in 22 genera and in seven classes. Aquat Microb Ecol 71:165–178
Telford WG, Moss MW, Morseman JP, Allnutt FCT (2001) Cryptomonad algal phycobiliproteins as fluorochromes for extracellular and intracellular antigen detection by flow cytometry. Cytometry 44:16–23
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York
Willén T (1962) Studies on the phytoplankton of some lakes connected with or recently isolated from the Baltic. Oikos 13:169–199
Zhu L (2015) Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew Sustain Energy Rev 41:1376–1384
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. This work was supported by the Maj and Tor Nessling Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization: E.P. and A.O.; Formal analysis and investigation: L.M.; Methodology: E.P.; Visualization: L.M.; Writing—original draft preparation: L.M.; Writing—review and editing: E.P. and A.O.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mercier, L., Peltomaa, E. & Ojala, A. Comparative analysis of phycoerythrin production in cryptophytes. J Appl Phycol 34, 789–797 (2022). https://doi.org/10.1007/s10811-021-02657-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02657-z