Abstract
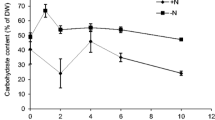
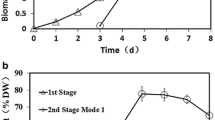
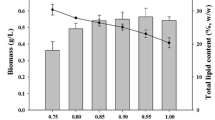
Microalgae are rich in various high-value compounds and thus have been proposed for use in biodiesel production and other industrial processes. Mixotrophy of microalgae can generate a high biomass and a high cell content of desirable compounds. To elucidate the probable relationship between pH drift, carbon source utilization, light intensity, and biomass accumulation in Chlorella cultivation, we compared the growth, pH drift, total biomass, and lipid and starch contents of Chlorella sorokiniana GXNN01 under different culture conditions. We also labeled the cell with 13C-labeled substrates. The results of pH drift and 13C labeling showed that Chlorella preferred acetate under high light, but glucose under low light. Glucose utilization resulted in decrease of pH, while acetate had the opposite effect. A combination of acetic acid and glucose as carbon source maintained the pH close to the initial value and significantly increased total biomass production. Cultures using acetic acid as carbon source or addition of sodium acetate under low light appeared to enhance total lipid content, while pH adjustment, addition of two carbon sources, or addition of sodium acetate under normal light (20 μmol photons m−2 s−1) resulted in high starch content. This knowledge will guide future Chlorella cultivation.





Similar content being viewed by others
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts—polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Arudchelvam Y, Nirmalakhandan N (2013) Energetic optimization of microalgal cultivation in photobioreactors for biodiesel production. Renew Energy 56:77–84
Benemann JR, Oswald WJ (1996) Systems and economic analysis of microalgae ponds for conversion of CO2 to biomass. Final report US DOE, Pittsburgh, USA, pp 1–201
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Branyikova I, Marsalkova B, Doucha J, Branyik T, Bisova K, Zachleder V, Vitova M (2011) Microalgae—novel highly efficient starch producers. Biotechnol Bioeng 108:766–776
Ceron Garcia MC, Camacho FG, Miron AS, Sevilla JMF, Chisti Y, Grima EM (2006) Mixotrophic production of marine microalga Phaeodactylum tricornutum on various carbon sources. J Microbiol Biotechnol 16:689–694
Feng B, Shao H, Cheng X (2006) Determination of formic acid and acetic acid concentrations in urine by high efficiency liquid chromatography. Chin J Health Lab Tech 16:207–208
Fischer E, Sauer U (2003) Metabolic flux profiling of Escherichia coli mutants in central carbon metabolism using GC-MS. Eur J Biochem 270:880–891
Garci MCC, Sevilla JMF, Fernandez FGA, Grima EM, Camacho FG (2000) Mixotrophic growth of Phaeodactylum tricornutum on glycerol: growth rate and fatty acid profile. J Appl Phycol 12:239–248
Garcia MCC, Miron AS, Sevilla JMF, Grima EM, Camacho FG (2005) Mixotrophic growth of the microalga Phaeodactylum tricornutum—influence of different nitrogen and organic carbon sources on productivity and biomass composition. Process Biochem 40:297–305
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A 54:1665–1669
Huan L, Xie X, Zheng Z, Sun F, Wu S, Li M, Gao S, Gu W, Wang G (2014) Positive correlation between PSI response and oxidative pentose phosphate pathway activity during salt stress in an intertidal macroalga. Plant Cell Physiol 55:1395–1403
Kong WB, Song H, Cao YT, Yang H, Hua SF, Xia CG (2011) The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. Afr J Biotechnol 10:11620–11630
Kotrbáček V, Doubek J, Doucha J (2015) The chlorococcalean alga Chlorella in animal nutrition: a review. J Appl Phycol 27:2173–2180
Lee YK, Ding SY, Hoe CH, Low CS (1996) Mixotrophic growth of Chlorella sorokiniana in outdoor enclosed photobioreactor. J Appl Phycol 8:163–169 doi:10.1007/bf02186320
Liang YN, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Maxwell DP, Falk S, Trick CG, Huner NP (1994) Growth at low temperature mimics high-light acclimation in Chlorella vulgaris. Plant Physiol 105:535–543
Nanchen A, Fuhrer T, Sauer U (2007) Determination of metabolic flux ratios from 13C-experiments and gas chromatography–mass spectrometry data. Methods Mol Biol 358:177–197
Perez-Garcia O, Escalante FM, de-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Qiao H, Wang G (2009) Effect of carbon source on growth and lipid accumulation in Chlorella sorokiniana GXNN01. Chin J Oceanol Limnol 27:762–768
Qiao H, Wang G, Zhang X (2009) Isolation and characterization of Chlorella sorokiniana GXNN01 (Chlorophyta) with the properties of heterotrophic and microaerobic growth. J Phycol 45:1153–1162
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev 35:265–278
Shi XM, Zhang XW, Chen F (2000) Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzym Microb Technol 27:312–318
Sijtsma L, Anderson AJ, Ratledge C (2010) Alternative carbon sources for heterotrophic production of docosahexaenoic acid by the marine alga Crypthecodinium cohnii. In: Ratledge C, Cohen Z (eds) Single cell oils: microbial and algal oils. AOCS Publishing, Pittsbourgh, pp 107–123
Van Wagenen J, De Francisci D, Angelidaki I (2015) Comparison of mixotrophic to cyclic autotrophic/heterotrophic growth strategies to optimize productivity of Chlorella sorokiniana. J Appl Phycol 27:1775–1782
Wang J, Curtis WR (2016) Proton stoichiometric imbalance during algae photosynthetic growth on various nitrogen sources: toward metabolic pH control. J Appl Phycol 28:43–52
Wen Z-Y, Chen F (2003) Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol Adv 21:273–294
Xie X et al (2015) Photorespiration participates in the assimilation of acetate in Chlorella sorokiniana under high light. New Phytol 52:434–441
Xiong W, Liu L, Wu C, Yang C, Wu Q (2010) 13C-tracer and gas chromatography–mass spectrometry analyses reveal metabolic flux distribution in the oleaginous microalga Chlorella protothecoides. Plant Physiol 154:1001–1011
Acknowledgments
This work was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (XDA11020404-1), National Natural Science Foundation of China (41406169), Postdoctoral Innovation Project Special Funds of Shandong Province (2014), and the National International cooperation project (2015DFG32160).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, A., Sun, L., Wu, S. et al. Utilization of glucose and acetate by Chlorella and the effect of multiple factors on cell composition. J Appl Phycol 29, 23–33 (2017). https://doi.org/10.1007/s10811-016-0920-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0920-6