Abstract
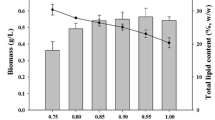
Heterotrophic culture of microalgae to develop methods of increasing biomass productivity and storage lipids has brought new insight to commercial biodiesel production. To understand the relationship between heterotrophy and lipid production, the effects of carbon sources on the growth and lipid accumulation of Chlorella sorokiniana GXNN01 was studied. The alga exhibited an increased growth rate in response to the addition of carbon sources, which reached the stationary phase after 48 h at 30°C. In addition, glucose and NaAc had a significant effect on the lipid accumulation during the early-stationary phase. Specifically, the lipid content was 0.237±0.026 g g−1 cell dry weight and 0.272±0.041 g L−1 when glucose was used as the carbon source, whereas the lipid content reached 0.287±0.018 g g−1 cell dry weight and 0.288±0.008 g L−1 when NaAc was used as the carbon source. The neutral lipid content was found to first decrease and then increase over time during the growth phase. A glucose concentration of 20 mmol L−1 gave the maximal lipid yield and the optimum harvest time was the early-stationary phase.
Similar content being viewed by others
References
An J Y, Sim S J, Lee J S, Kim B W. 2003. Hydrocarbon production from secondarily treated piggery wastewater by the green alga Botryococcus braunii. J. Appl. Phycol., 15: 185–191.
Angelo C P, Lilian L N G, Michelle J C R, Núbia M R, Ednildo A T, Wilson A L, Pedro A P P, Jailson B A. 2005. Biodiesel: An Overview. J. Braz. Chem. Soc., 16: 1 313–1 330.
Barclay W, Meager K, Abril J. 1994. Heterotrophic production of long chain omega-3 fatty acids utilizing algae and algae-like microorganisms. J. Appl. Phycol., 6: 123–129.
Behrens P W, Kyle D J. 1996. Microalgae as a source of fatty acids. J. Food Lipids, 3: 259–272.
Bligh E G, Dyer W J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol., 37: 911–917.
Borowitzka M A. 1995. Microalgae source of pharmaceuticals and other biologically active compounds. J. Appl. Phycol., 7: 3–15.
Borowitzka M A. 1999. Commercial production of microalgae: ponds, tanks, tubes and fermenters. J. Biotechnol., 70: 313–321.
Buchanan B B, Gruissem W, Jones R L. 2000. Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists. USA. p. 630–728.
Cao C, Sun S, Mai K, Liang Y. 2000. Fatty acid composition and total lipid content of 30 strains of marine green algae. Journal of Ocean University of Qingdao, 30(3): 428–434.
Chen F. 1996. High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol., 14: 421–426.
Chisti Y. 2007. Biodiesel from microalgae. Biotechnol. Adv., 25: 294–306.
Cooksey K E, Guckert J B, Williams S A, Collis P R. 1987. Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J. Microbiol. Meth., 6: 333–345.
Elsey D, Jameson D, Raleigh B, Cooney M J. 2007. Fluorescent measurement of microalgal neutral lipids. J Microbiol. Meth., 68: 639–642.
Greenspan P, Fowler S D. 1985. Spectrofluorometric studies of the lipid probe, nile red. J. Lipid. Res., 26: 781–789.
Greenspan P, Mayer E P, Fowler S D. 1985. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol., 100: 963–973.
Kyle D J. 1992. Production and use of lipids from microalgae. Lipid Technol., 4: 59–64.
Lee S J, Yoon B D, Oh H M. 1998. Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol. Tech., 12: 553–556.
Liu Z, Wang G, Zhou B. 2008. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresource Technol., 99: 4 717–4 722.
Miao X, Wu Q. 2006. Biodiesel production from heterotrophic microalgal oil. Bioresource Technol., 97: 841–846.
Miao X, Wu Q, Yang C. 2004. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis, 71: 855–863.
Nichols H W. 1973. Growth Media-Freshwater. In: Stein J ed. Handbook of Phycological Methods, Culture Methods and Growth Measurements. Camb. Univ. Press, UK, p. 7–24.
Running J A, Huss R J, Olson P T. 1994. Heterotrophic production of ascorbic acid by microalgae. J. Appl. Phycol., 4: 99–104.
Sheehan J, Dunahay T, Benemann J, Roessler P. 1998. A Look Back at the U.S. Department of Energy’s Aquatic Species Program—Biodiesel from Algae. Golden, USA. p.12–13.
Spolaore P, Cassan C J, Duran E, Isambert A. 2006. Commercial applications of microalgae. J. Biosci. Bioeng., 101: 87–96.
Stewart W D P. 1974. Algae Physiology and Biochemistry. In: Burnett J H, Baker H G, Beevers H, Whatley F R eds. Botanical Monographys. Blackwell Scientific Publications, London, UK. p. 505–508.
Tan C, Johns M. 1991. Fatty acid production by heterotrophic Chlorella saccharophila. Hydrobiologia, 215: 13–19.
Volkman J K, Jeffrey S W, Nichds P D. 1989. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Bial. Ecal., 128: 219–240.
Vicente G, Martinez M, Aracil J. 2004. Integrated biodiesel production: a comparison of different homogeneous catalysts systems. Bioresource Technol., 92: 297–305.
Wen Z, Chen F. 2003. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol. Adv., 21: 273–294.
Williams P B. 2007. Biofuel: microalgae cut the social and ecological costs. Nature, 450: 478.
Xiong W, Li X, Xiang J, Wu Q. 2008. High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl. Microbiol. Biotechnol., 78: 29–36.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National High Technology Research and Development Program of China (863 Program) (No. 2006AA05Z112), the Key Project of Science and Technology for Supporting Tianjin Development (No. 2007LS700310), and the Knowledge Innovation Project of Chinese Academy of Sciences (No. KGCX2-YW-374-3)
Rights and permissions
About this article
Cite this article
Qiao, H., Wang, G. Effect of carbon source on growth and lipid accumulation in Chlorella sorokiniana GXNN01. Chin. J. Ocean. Limnol. 27, 762–768 (2009). https://doi.org/10.1007/s00343-009-9216-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-009-9216-x