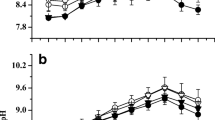
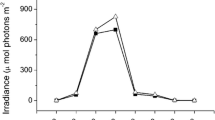
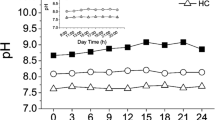
Abstract
Growth and photosynthesis of cultivated seaweeds usually suffer carbon limitation during cultivation in the field. Pyropia haitanensis and Gracilaria lemaneiformis, collected from Nan’ao Island, Shantou, China, were cultured under ambient carbon and decreased carbon supply, with ambient sunlight and decreased sunlight conditions, aiming to investigate how the decreased carbon supply and sunlight conditions affect growth and photosynthesis in these two maricultured seaweed species. Decreased carbon supply significantly lowered the relative growth rate (RGR), quantum efficiency of open PS II (F v′/F m′), maximum photosynthetic electron transport rate (rETRm), and NO3 − uptake rate in both of the two seaweeds. Under ambient sunlight condition, the RGR of the P. haitanensis and G. lemaneiformis grown at decreased carbon supply was reduced about 83 and 95 %, respectively, compared with the algae grown at ambient carbon condition. The RGR, F v′/F m′, and NO3 − uptake rate were higher in P. haitanensis but were lower in G. lemaneiformis, with under decreased sunlight compared to ambient sunlight. The results indicated that decreased carbon supply reduced growth and PS II activity in both of the seaweeds, with the reduction being greater in G. lemaneiformis than in P. haitanensis. Additionally, G. lemaneiformis was adapted to grow at relative higher light conditions than P. haitanensis.





Similar content being viewed by others
References
Andría JR, Brun FG, Pérez-Lloréns JL, Vergara JJ (2001) Acclimation responses of Gracilaria sp. (Rhodophyta) and Enteromorpha intestinalis (Chlorophyta) to changes in the external inorganic carbon concentration. Bot Mar 44:361–370
Baker NR (1991) A possible role for Photosystem II in environmental perturbations of photosynthesis. Physiol Plant 81:563–570
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust J Mar Freshwat Res 36:785–792
Beer S, Mtolera M, Lyimo T, Björk M (2006) The photosynthetic performance of the tropical seagrass Halophila ovalis in the upper intertidal. Aquat Bot 84:367–371
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Campbell SJ, Kerville SP, Coles RG, Short F (2008) Photosynthetic responses of subtidal seagrasses to a daily light cycle in Torres Strait: a comparative study. Cont Shelf Res 28:2275–2281
Chow F, de Oliveira MC, Pedersén M (2004) In vitro assay and light regulation of nitrate reductase in red alga Gracilaria chilensis. J Plant Physiol 161:769–776
Chung IK, Oak JH, Lee JA, Shin JA, Kim GJ, Park KS (2013) Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES J Mar Sci 11:1–7
Durchan M, Vacha F, Krieger-Liszkay A (2001) Effects of severe CO2 starvation on the photosynthetic electron transport chain in tobacco plants. Photosynth Res 68:203–213
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell Science, Malden, pp 128–135
Fei XG (2004) Solving the coastal eutrophication problem by large scale seaweed cultivation. Hydrobiologia 512:145–151
Friedlander M, Levy I (1995) Cultivation of Gracilaria in outdoor tanks and ponds. J Appl Phycol 7:315–324
García-Sânchez MJ, Fernândez JA, Niell FX (1994) Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 194:55–61
Gordillo FJL, Niell FX, Figueroa FL (2001) Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213:64–70
Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH (2012) Effects of climate change on global seaweed communities. J Phycol 48:1064–1078
Harrison PJ (1988) Determining phosphate uptake rates of phytoplankton. In: Lobban CS, Chapman DJ, Kremer BP (eds) Experimental phycology: a laboratory manual. Cambridge University Press, New York, pp 186–195
Israel A, Friedlander M (1998) Inorganic carbon utilisation and growth abilities in the marine red macroalga Gelidiopsis sp. Isr J Plant Sci 46:117–124
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Oceanography 21:540–547
Koch M, Bowes G, Ross C, Zhang XH (2013) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Chang Biol 19:103–132
Lillo C (1994) Light regulation of nitrate reductase in green leaves of higher plants. Physiol Plant 90:616–620
Lopes PF, Oliveira MC, Colepicolo P (1997) Diurnal fluctuation of nitrate reductase activity in the marine red alga Gracilaria tenuistpitata (Rhodophyta). J Phycol 33:225–231
Magnusson G (1997) Diurnal measurements of Fv/Fm used to improve productivity estimates in macroalgae. Mar Biol 130:203–208
McMinn A, Ryan K, Gademann R (2003) Diurnal changes in photosynthesis of Antarctic fast ice algal communities determined by pulse amplitude modulation fluorometry. Mar Biol 143:359–367
Mercado JM, Javier F, Gordillo L, Niell FX, Figueroa FL (1999) Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J Appl Phycol 11:455–461
Parsons TR, Strickland JDH (1963) Discussion of spectrophotometric determination of marine plant pigments, with revised equation for ascertaining chlorophylls and carotenoids. J Mar Res 21:155–163
Porra RJ (2005) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Ralph PJ, Gademann R, Larkum AWD, Schreiber U (1999) In situ underwater measurements of photosynthetic activity of coral zooxanthellae and other reef-dwelling dinoflagellate endosymbionts. Mar Ecol Prog Ser 180:139–147
Richards DK, Hurd CL, Pritchard DW, Wing SR, Hepburn CD (2011) Photosynthetic response of monospecific macroalgal stands to density. Aquat Biol 13:41–49
Sagert S, Forster RM, Feuerpfeil P, Schubert H (1997) Daily course of photosynthesis and photoinhibition in Chondrus crispus (Rhodophyta) from different shore levels. Eur J Phycol 32:363–371
Serôdio J, Vieira S, Cruz S (2008) Photosynthetic activity, photoprotection and photoinhibition in intertidal microphytobenthos as studied in situ using variable chlorophyll fluorescence. Cont Shelf Res 28:1363–1375
Strickland JDH, Parsons TR (1972) A Practical Handbook of Seawater Analysis. Fisheries Research Board of Canada, Ottawa
Talarico L, Maranzana G (2000) Light and adaptive responses in red macroalgae: an overview. J Photochem Photobiol B 56:1–11
Yang H, Zhou Y, Mao Y, Li X, Liu Y, Zhang F (2005) Growth characters and photosynthetic capacity of Gracilaria lemaneiformis as a biofilter in a shellfish farming area in Sanggou Bay, China. J Appl Phycol 17:199–206
Yang Y, Liu Q, Chai Z, Tang Y (2015) Inhibition of marine coastal bloom-forming phytoplankton by commercially cultivated Gracilaria lemaneiformis (Rhodophyta). J Appl Phycol 27:2341–2352
Zou DH (2005) Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250:726–735
Zou DH (2014) The effects of severe carbon limitation on the green seaweed, Ulva conglobata (Chlorophyta). J Appl Phycol 26:2417–2424
Zou DH, Gao KS (2002) Effects of desiccation and CO2 concentrations on emersed photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta), a species farmed in China. Eur J Phycol 37:587–592
Zou DH, Gao KS (2009) Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia 48:510–517
Zou DH, Gao KS (2010) Physiological responses of seaweeds to elevated atmospheric CO2 concentrations. In: Israel A, Einav R, Seckbach J (eds) Seaweeds and their role in globally changing environment. Springer, Dordrecht, pp 115–126
Zou DH, Xia JR, Yang YF (2004) Photosynthetic use of exogenous inorganic carbon in the agarophyte Gracilaria lemaneiformis (Rhodophyta). Aquaculture 237:421–431
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. U1301235 and No. 41276148) and Guangdong Science and Technology Bureau (2015A020216004). The authors would like to thank Yayun Deng and Jiejun Zhang for assistance with the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, H., Zou, D. & Chen, B. Effects of lowered carbon supplies on two farmed red seaweeds, Pyropia haitanensis (Bangiales) and Gracilaria lemaneiformis (Gracilariales), grown under different sunlight conditions. J Appl Phycol 28, 3469–3477 (2016). https://doi.org/10.1007/s10811-016-0882-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0882-8