Abstract
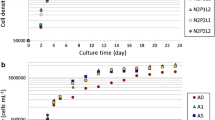
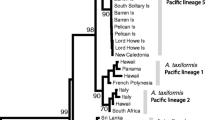
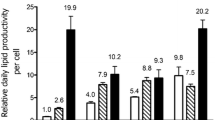
Diatoms (Bacillariophyta) characteristically accumulate valuable storage lipids throughout their entire life cycle and therefore are an appealing choice to be used as a feedstock for commercial products, like biodiesel. However, the incredible diversity of diatoms poses a substantial challenge when attempting to select the best species for commercial development. The present study compiled previously published data regarding diatom lipid content under nutrient-replete and nutrient(s)-deplete conditions as well as generating novel lipid and growth data from ten species of diatoms within lineages that have been historically under-examined. In this study, lipids were extracted via chloroform-methanol and quantified as percent dry weight and then analyzed for a phylogenetic signal by comparing the variability between lineages to the variability within lineages in an attempt to identify the lineages with the greatest lipid content. A nested ANOVA revealed that there was greater variability between lineages than within lineages when cultures were grown in a silica-deplete medium; the Biddulphiophycidae lineage accumulated a significantly lower amount of lipids than other lineages. The taxa examined in the present study were then paired with the data gathered from the literature and examined for a phylogenetic signal using previously described methods. In this analysis, a statistically significant phylogenetic signal was detected for nutrient(s)-deplete growth experiments (P = 0.013). These results suggest that under nutrient(s)-deplete conditions, lipid content is, in part, a heritable, evolutionary trait, and therefore, an evolutionary-based approach may be used to facilitate species selection by identifying the differences in lipid content between lineages.






Similar content being viewed by others
References
Ashworth MP, Nakov T, Theriot EC (2013) Revisiting Ross and Sims (1971): toward a molecular phylogeny of the Biddulphiaceae and Eupodiscaceae (Bacillariophyceae). J Phycol 49:1207–1222
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits and more labile. Evolution 57:717–745
Ceron Garcia MC, Fernandez Sevilla JM, Acien Fernandez FG, Molina Grima E, Garcia Camacho F (2000) Mixotrophic growth of Phaeodactylum tricornutum on glycerol: growth rate and fatty acid profile. J Appl Phycol 12:239–248
Chen YC (2012) The biomass and total lipid content and composition of twelve species of marine diatoms cultured under various environments. Food Chem 131:211–219
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Coombs J, Darley WM, Holm-Hansen O, Volcani BE (1967) Studies on the biochemistry and fine structure of silica shell formation in diatoms. Chemical composition of Navicula pelliculosa during silicon-starvation synchrony. Plant Physiol 42:1601–1606
De la Pena MR (2007) Cell growth and nutritive value of the tropical benthic diatoms, Amphora sp., at varying levels of nutrient and light intensity, and different culture locations. J Appl Phycol 19:647–655
Demirbas A, Demirbas MF (2011) Importance of algae oil as a source of biodiesel. Energy Convers Manag 52:163–170
Fabris M, Matthijs M, Rombauts S, Vyverman W, Goossens A, Baart G (2012) The metabolic blueprint of Phaeodactylum tricornutum reveals a eukaryotic Entner-Doudoroff glycolytic pathway. Plant J 70:1004–1014
Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJR (2004) The evolution of modern eukaryotic phytoplankton. Science 305:354–360
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fourtainer E, Kociolek JP (1999) Catalogue of the diatom genera. Diatomol Res 14:1–190
Gatenby CM, Orcutt DM, Kreeger DA, Parker BC, Jones VA, Neves RJ (2003) Biochemical composition of three algal species proposed as food for captive freshwater mussels. J Appl Phycol 15:1–11
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224
Graham JM, Graham LE, Zulkifly SB, Pfleger BF, Hoover SW, Yoshitani J (2011) Freshwater diatoms as a source of lipids for biofuels. J Ind Microbiol Biotechnol 39:419–428
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum, New York, pp 29–60
Hildebrand M, Aubrey DK, Smith SR, Traller JC, Abbriano R (2012) The place of diatoms in the biofuels industry. Biofuels 3:221–240
Hillebrand H, Durselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Hoppenrath M, Elbrachter M, Drebes G (2009) Marine phytoplankton: selected microphytoplankton species from the North Sea around Helgoland and Sylt. E Schweizerbart, Stuttgart
Jorgensen EG (1977) Photosynthesis. In: Werner D (ed) The biology of diatoms. University of California Press, Berkeley, pp 150–168
Krammer K, Lange-Bertalot H (2008) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasser Flora von Mitteleuropa, Band 2/3. Spektrum Akademischer Verlag, Heidelberg
Laurens LML, Quinn M, Wychen SV, Templeton DW, Wolfrum EJ (2012) Accurate and reliable quantification of total microalgal fuel potential as fatty acid methyl esters by in situ transesterification. Anal Bioanal Chem 403:167–178
Mansour MP, Frampton DMF, Nichols PD, Volkman JK, Blackburn SI (2005) Lipid and fatty acid yield of nine stationary-phase microalgae: applications and unusual C24-C28 polyunsaturated fatty acids. J Appl Phycol 17:287–300
Nagle N, Lemke P (1990) Production of methyl ester fuel from microalgae. Appl Biochem Biotechnol 24–25:355–361
Norton TA, Melkonian M, Andersen RA (1996) Algal biodiversity. Phycologia 35:308–326
Orcutt DM, Patterson GW (1975) Sterol, fatty acid and elemental composition of diatoms grown in chemically defined media. Comp Biochem Physiol 50B:579–583
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Renaud SM, Parry DL, Thinh LV (1994) Microalgae for use in tropical aquaculture I: gross chemical and fatty acid of twelve species of microalgae from the Northern Territory, Australia. J Appl Phycol 6:337–345
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bionini G, Tredici MR (2008) Mircoalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Round FE, Crawford RM, Mann DG (1990) The diatoms: biology and morphology of the genera. Cambridge University Press, Cambridge
Scala S, Bowler C (2001) Molecular insights into the novel aspects of diatom biology. Cell Mol Life Sci 58:1666–1673
Scholz B, Liebezeit G (2013) Biochemical characterization and fatty acid profiles of 25 benthic marine diatoms isolated from the Solthorn tidal flat (southern North Sea). J Appl Phycol 25:453–465
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program: biodiesel from Algae. Close-Out report. National Renewable Energy Lab, Department of Energy, Golden, Colorado, U.S.A. Report number NREL/TP-580-24190
Sims PA, Mann DG, Medlin LK (2006) Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia 45:361–402
Theriot E (1987) Principal component analysis and taxonomic interpretation of environmentally related variation in silicification in Stephanodiscus (Bacillariophyceae). Br Phycol J 22:359–373
Theriot EC, Ashworth M, Ruck E, Nakov T, Jansen RK (2010) A preliminary multigene phylogeny of the diatoms (Bacillariophyta): challenges for future research. Plant Ecol Evol 143:278–296
Tomas CR (1997) Identifying marine phytoplankton. Academic, Boston
US DOE (Department of Energy) (2010) National algal biofuels technology roadmap. US Department of Energy, Office of Energy Efficiency and Renewable Energy, Biomass Program
Wang C, Kong H, He S, Zheng X, Li C (2009) The inverse correlation between growth rate and cell carbohydrate content of Microcystis aeuginosa. J Appl Phycol 22:105–107
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fields, F.J., Kociolek, J.P. An evolutionary perspective on selecting high-lipid-content diatoms (Bacillariophyta). J Appl Phycol 27, 2209–2220 (2015). https://doi.org/10.1007/s10811-014-0505-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0505-1