Abstract
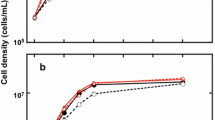
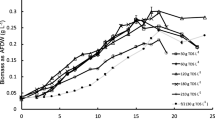
Until recently, biodiesel production has been derived from terrestrial plants such as soybean and canola, leading to competition between biodiesel production and agricultural production for source materials. Microalgae have the potential to synthesize 30 times more oil per hectare than terrestrial plants without competing for agricultural land. We examined four genera (Cyclotella, Aulacoseira, Fragilaria, Synedra) of common freshwater diatoms (Bacillariophyceae) for growth and lipid content in defined medium (sD11) that replicates hypereutrophic conditions in lakes and wastewater treatment plant effluents and optimized the medium for silicon content. Cyclotella and Aulacoseira produced the highest levels of total lipids, 60 and 43 μg total lipids/ml, respectively. Both diatoms are rich in fatty acids C14, C16, C16:1, C16:2,7,10, and C22:5n3. Of the diatoms examined, Cyclotella reached the highest population density (>2.5 × 106 cells/ml) in stationary phase when many of the cells appeared to be filled entirely with oil. Silicon enrichment studies indicated that for optimal utilization of phosphorus and nitrogen by diatoms growing in wastewater effluent, the amount of silicon present or added to the effluent should be 17.5 times the mass of phosphorus in the effluent. With high growth rates, high lipid contents, and rapid settling rates, Cyclotella and Aulacoseira are candidates for biodiesel production.






Similar content being viewed by others
References
Alonso DL, Segura del Castillo CI, Grima EM, Cohen Z (1996) First insights into improvement of eicosapentaenoic acid content in Phaeodactylum tricornutum (Bacillariophyceae) by induced mutagenesis. J Phycol 32:339–345
Baliga R, Powers SE (2010) Sustainable algae biodiesel production in cold climates. Int J Chem Eng. doi:10.1155/2010/102179
Brown MR, Dunstan GA, Norwood SJ, Miller KA (1996) Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J Phycol 32:64–73
Cerón García MC, Fernández Sevilla JM, Acien Fernandez FG, Molina Grima E, García Camcho F (2000) Mixotrophic growth of Phaeodactylum tricornutum on glycerol: growth rate and fatty acid profile. J Appl Phycol 12:239–248
Cerón García MC, García Camacho F, Sánchez Mirón A, Fernández Sevilla JM, Chisti Y, Molina Grima E (2006) Mixotrophic production of marine microalga Phaeodactylum tricornutum on various carbon sources. J Microbiol Biotechnol 16:689–694
Chen G-Q, Jiang Y, Chen F (2007) Fatty acid and lipid class composition of the eicosapentaenoic acid-producing microalga, Nitzschia laevis. Food Chem 104:1580–1585
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. doi:10.1016/j.biotechadv.2007.02.001
Clarens AF, Resurreccion EP, White MA, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44:1813–1819. doi:10.1021/es902838n
De la Pena MR (2007) Cell growth and nutritive value of the tropical benthic diatom, Amphora sp., at varying levels of nutrients and light intensity, and different culture locations. J Appl Phycol 19:647–655
Fabregas J, Otero A, Dominguez A, Patino M (2001) Growth rate of the microalga Tetraselmis suecica changes the biochemical composition of Artemia species. Mar Biotechnol 3:256–263
Fuentes-Grünewald C, Garcés E, Rossi S, Camp J (2009) Use of the dinoflagellate Karlodinium veneficum as a sustainable source of biodiesel production. J Ind Microbiol Biotechnol 36:1215–1224. doi:10.1007/s10295-009-0602-3
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274. doi:10.1007/s10295-008-0495-6
Graham JM, Lembi CA, Adrian HL, Spencer DF (1995) Physiological responses to temperature and irradiance in Spirogyra (Zygnematales, Charophyceae). J Phycol 31:531–540
Graham JM, Auer MT, Canale RP, Hoffmann JP (1982) Ecological studies and mathematical modeling of Cladophora in Lake Huron: 4. Photosynthesis and respiration as functions of light and temperature. J Great Lakes Res 8:100–111
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507. doi:10.1007/s10811-008-9392-7
Guerin M, Huntley ME, Olaizola M (2003) Hematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21:210–216
Heredia-Arroyo T, Wei W, Hu B (2010) Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl Biochem Biotechnol 162:1978–1995. doi:10.1007/s12010-010-8974-4
Hoffmann JP (1998) Wastewater treatment with suspended and nonsuspended algae. J Phycol 34:757–763
Hoover SW, Marner WD II, Brownson AK, Lennen RM, Wittkopp TM, Yoshitani J, Zulkifly S, Graham LE, Chaston S, McMahon KD, Pfleger BF (2011) Bacterial production of free fatty acids from freshwater, macroalgal cellulose. Appl Microbiol Biotechnol 91:435–446. doi:10.1007/s00253-011-3344-x
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huntley ME, Redalje DG (2007) CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitig Adapt Strat Global Change 12:573–608. doi:10.1007/s11027-006-7304-1
Koh LP (2007) Potential habitat and biodiversity losses from intensified biodiesel feedstock production. Conserv Biol 21:1373–1375
Lee YK (2001) Microbial mass culture systems and methods: their limitations and potential. J Appl Phycol 13:307–315
Lennen RM, Braden DJ, West RM, Dumesic JA, Pfleger BF (2010) A process for microbial hydrocarbon synthesis: overproduction of fatty acids in Escherichia coli and catalytic conversion to alkanes. Biotechnol Bioeng 106:193–202. doi:10.1002/bit.22660
Li X, Xu H, Wu Q (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98:764–771
Liu X, Sheng J, Curtis R III (2011) Fatty acid production in genetically modified cyanobacteria. PNAS 108:6899–6904. doi:10.1073/pnas.1103014108
Lowe RL (1975) Comparative ultrastructure of the valves of some Cyclotella species (Bacillariophyceae). J Phycol 11:415–424
McGinnis KM, Dempster TA, Sommerfeld MR (1997) Characterization of the growth and lipid content of the diatom Chaetoceros muelleri. J Phycol 9:19–24
Metzger P, Largeau C (2005) Botryococcus braunii: a rich source for hydrocarbons and related ether lipids. Appl Microbiol Biotechnol 66:486–496. doi:10.1007/s00253-004-1779-z
Miron AS, Garcia MCC, Gomez AC, Camacho FG, Grima EM, Chisti Y (2003) Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem Engin J 16:287–297. doi:10.1016/s1369-703x(03)00072-x
Patrick R, Reimer CW (1966) The diatoms of the United States, vol 1. The Academy of Natural Sciences of Philadelphia, Sutter House, Lititz
Pew Center on Global Climate Change (2011) Transportation Overview. http://www.pewclimate.org/technology/overview/transportation
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648. doi:10.1007/s00253-004-1647-x
Reynolds CS (2006) Ecology of phytoplankton. Cambridge University Press, Cambridge
Richmond A (1990) Large scale microalgal culture and applications. Progress Phycol Res 7:269–330
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2008) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Round FE, Crawford RM, Mann DG (1990) The diatoms: biology and morphology of the genera. Cambridge University Press, Cambridge
Shafik HM, Herodek S, Vörös L, Présing M, Kiss KT (1997) Growth of Cyclotella meneghiniana Kutz I. Effects of temperature, light and low rate of nutrient supply. Annls Limnol 33:139–147
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae. Close-out report. National Renewable Energy Lab, Department of Energy, Golden, Colorado, U.S.A. Report number NREL/TP-580-24190, dated July 1998
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96. doi:10.1263/jbb.101.87
USEPA (2008) Clean watersheds needs survey 2004, report to Congress. Appendix C, Table C-3. http://water.epa.gov/scitech/datait/databases/cwns/toc.cfm
Vonshak A, Cheung SM, Chen F (2000) Mixotrophic growth modifies the response of Spirulina (Arthrospira) platensis (Cyanobacteria) cells to light. J Phycol 36:675–679
Watson SB, McCauley E, Downing JA (1997) Patterns in phytoplankton taxonomic composition across temperate lakes of different nutrient status. Limnol Oceanogr 42:487–495
Wu H, Volponi JV, Oliver AE, Parikh AN, Simmons BA, Singh S (2011) In vivo lipidomics using single-cell Raman spectroscopy. Proc Natl Acad Sci 108:3809–3814. doi:10.1073/pnas.1009043108
Xu H, Miao XL, Wu QY (2006) High-quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Yongmanitchai W, Ward OP (1991) Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl Environ Microbiol 57:419–425
Yu ET, Zendejas FJ, Lane PD, Gaucher S, Simmons BA, Lane TW (2009) Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Bacillariophyceae) during starvation. J Appl Phycol 21:669–681. doi:10.1007/s10811-008-9400-y
Acknowledgments
The results reported here represent part of a larger study funded by the Wisconsin State Office of Energy Independence.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graham, J.M., Graham, L.E., Zulkifly, S.B. et al. Freshwater diatoms as a source of lipids for biofuels. J Ind Microbiol Biotechnol 39, 419–428 (2012). https://doi.org/10.1007/s10295-011-1041-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-1041-5