Abstract
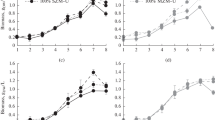
The axenic strain BOT-22 (race B) of Botryococcus braunii was investigated for the influence of iron and glucose on its morphology to reveal the relationship with growth and oil production. The average size of the iron-rich cells was larger than that of iron-limited cells. The shapes of iron-rich cells were elliptical and that of iron-limited cells were conical. Several cells were tightly connected to form large colonies in the iron-rich culture; however, only a few cells were sparsely arranged to form the small colonies in the iron-limited culture. Glucose addition to the iron-rich culture further increased the size of the cells and colonies. The growth increased in the following order: iron-limited, iron-rich, and iron-rich with glucose cultures. The same was observed for the volume of hydrocarbons in iron-rich cultures. It was also speculated that the shapes of cells and the amount of extracellular oil are most likely related to colony size and form.






Similar content being viewed by others
References
Allard B, Casadevall E (1990) Carbohydrate composition and characterization of sugars from the green microalga Botryococcus braunii. Phytochemistry 29:1875–1878
Beakes GW, Cleary AL (1999) Visualization of plastids and lipophilic components in living colonies of a wild strain of the hydrocarbon-forming, green alga Botryococcus by laser scanning confocal microscopy. J Appl Phycol 10:435–466
Berkaloff C, Rousseau B, Couté A, Casadevall E, Metzger P, Chirac C (1984) Variability of cell wall structure and hydrocarbon type in different strains of Botryococcus braunii. J Phycol 20:377–389
Casadevall E, Dif D, Largeau C, Gudin C, Chaumont D, Desanti O (1985) Studies on batch and continuous culture of Botryococcus braunii: hydrocarbon production in relation to physiological state, cell ultrastructure, and phosphate nutrition. Biotechnol Bioeng 27:286–295
Dayananda C, Sarada R, Bhattacharya S, Ravishankar GA (2005) Effect of media and culture conditions on growth and hydrocarbon production by Botryococcus braunii. Process Biochem 40:3125–3131
Hirose H, Ogasawara N (1977) Fine structural evidence for the systematic position of Botryococcus braunii Kutzing as a member of Chlorophyceae. Bull Jpn Soc Phycol 25:61–69
Komarek J, Marvan P (1992) Morphological differences in natural populations of the genus Botryococcus (Chlorophyceae). Arch Protistenkd 141:65–100
Largeau C, Casadeval E, Berkaloff C, Dhamelincourt P (1980) Sites of accumulation and composition of hydrocarbons in Botryococcus braunii. Phytochemistry 19:1043–1051
Metzger P, Allard B, Casadevall E, Berkaloff C, Couté A (1990) Structure and chemistry of a new chemical race of Botryococcus braunii (Chlorophyceae) that produces lycopadiene, a tetraterpenoid hydrocarbon. J Phycol 26:258–266
Plain N, Largeau C, Derenne S, Coutè A (1993) Variabilité morphologique de Botryococcus braunii (Chlorococcales, Chlorophyta): corrélations avec les conditions de croissance et la teneur en lipides. Phycologia 32:259–265
Simpson AJ, Zang X, Kramer R, Hatcher PG (2003) New insights on the structure of algaenan from Botryococcus braunii race A and its hexane insoluble botryals based on multidimensional NMR spectroscopy and electrospray–mass spectrometry techniques. Phytochemistry 62:783–796
Tanoi T, Kawachi M, Watanabe MM (2011) Effects of carbon source on growth and morphology of Botryococcus braunii. J Appl Phycol 23:25–33
Wake LV, Hillen LW (1980) Study of a “Bloom” of the oil-rich alga Botryococcus braunii in the Darwin River Reservoir. Biotech Bioeng 12:1637–1656
Weiss TL, Roth R, Goodson C, Vitha S, Black I, Azadi P, Rusch J, Holzenburg A, Devarenne TP, Goodenough U (2012) Colony organization in the green alga Botryococcus braunii (Race B) is specified by a complex extracellular matrix. Eukaryot Cell 11:1424–1440
Wolf F, Cox ER (1981) Ultrastructure of active and resting colonies of Botryococcus braunii (Chlorophyceae). J Phycol 17:395–405
Yeesang C, Cheirslip B (2010) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Biores Technol 102:3034–3040
Zhang K, Kojima E (1998) Effect of light intensity on colony size of microalga Botryococcus braunii in bubble column photobioreactors. J Ferment Bioeng 86:575–576
Acknowledgments
We thank Prof. Kunimitsu Kaya of the University of Tsukuba, Japan, for providing data on the analysis of hydrocarbons in strain BOT-22. This work was financially supported by the Core Research of Evolutional Science & Technology program from the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanoi, T., Kawachi, M. & Watanabe, M.M. Iron and glucose effects on the morphology of Botryococcus braunii with assumption on the colony formation variability. J Appl Phycol 26, 1–8 (2014). https://doi.org/10.1007/s10811-013-0026-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0026-3