Abstract
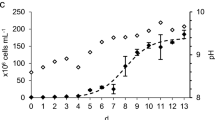
There has been considerable interest in cultivation of green microalgae (Chlorophyta) as a source of lipid that can alternatively be converted to biodiesel. However, almost all mass cultures of algae are carbon-limited. Therefore, to reach a high biomass and oil productivities, the ideal selected microalgae will most likely need a source of inorganic carbon. Here, growth and lipid productivities of Tetraselmis suecica CS-187 and Chlorella sp were tested under various ranges of pH and different sources of inorganic carbon (untreated flue gas from coal-fired power plant, pure industrial CO2, pH-adjusted using HCl and sodium bicarbonate). Biomass and lipid productivities were highest at pH 7.5 (320 ± 29.9 mg biomass L−1 day−1and 92 ± 13.1 mg lipid L−1 day−1) and pH 7 (407 ± 5.5 mg biomass L−1 day−1 and 99 ± 17.2 mg lipid L−1 day−1) for T. suecica CS-187 and Chlorella sp, respectively. In general, biomass and lipid productivities were pH 7.5 > pH 7 > pH 8 > pH 6.5 and pH 7 > pH 7.5 = pH 8 > pH 6.5 > pH 6 > pH 5.5 for T. suecica CS-187 and Chlorella sp, respectively. The effect of various inorganic carbon on growth and productivities of T. suecica (regulated at pH = 7.5) and Chlorella sp (regulated at pH = 7) grown in bag photobioreactors was also examined outdoor at the International Power Hazelwood, Gippsland, Victoria, Australia. The highest biomass and lipid productivities of T. suecica (51.45 ± 2.67 mg biomass L−1 day−1 and 14.8 ± 2.46 mg lipid L−1 day−1) and Chlorella sp (60.00 ± 2.4 mg biomass L−1 day−1 and 13.70 ± 1.35 mg lipid L−1 day−1) were achieved when grown using CO2 as inorganic carbon source. No significant differences were found between CO2 and flue gas biomass and lipid productivities. While grown using CO2 and flue gas, biomass productivities were 10, 13 and 18 %, and 7, 14 and 19 % higher than NaHCO3, HCl and unregulated pH for T. suecica and Chlorella sp, respectively. Addition of inorganic carbon increased specific growth rate and lipid content but reduced biomass yield and cell weight of T. suecica. Addition of inorganic carbon increased yield but did not change specific growth rate, cell weight or content of the cell weight of Chlorella sp. Both strains showed significantly higher maximum quantum yield (Fv/Fm) when grown under optimum pH.




Similar content being viewed by others
References
Australian Bureau of Meteorology (2012) Average annual daily Australian solar exposure. http://reg.bom.gov.au/jsp/ncc/climate_averages/ solar-exposure/index.jsp. Accessed: 8th of January 2012
Benemann J (1992) Microalgae aquaculture feeds. J Appl Phycol 4:233–245
Benemann JR, Oswald WJ (1996) Systems and economic analysis of microalgae ponds for conversion of CO2 to biomass. Pittsburgh Energy Technology Center. Final report to the Department of Energy, Pittsburgh, p 201
Benemann JR, Goebel RP, Weismann JC, Augenstein DC (1982) Microalgae as a source of liquid fuels. Final technical report to US Department of Energy. US Department of Energy, Washington DC
Borowitzka MA (1982) Mechanisms in algal calcification. Prog Phycol Res 1:137–177
Borowitzka MA (1997) Microalgae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Borowitzka MA (1998) Limits to growth. In: Wong YS, Tam NFY (eds) Wastewater treatment with algae. Springer, Berlin, pp 203–226
Borowitzka MA, Larkum AWD (1976) Calcification in the green alga Halimeda II. The exchange of Ca2+ and the occurrence of age gradients in the calcification and photosynthesis. J Exp Bot 27:864–878
Borowitzka MA, Moheimani NR (2010) Sustainable biofuels from algae. Mitig Adapt Strat Global Change. doi:10.1007/s11027-010-9271-9
Brewer PG, Goldman JC (1976) Alkalinity changes generated by phytoplankton growth. Limnol Oceanogr 21:108–117
Campbell PK, Beer T, Batten D (2010) Life cycle assessment of biodiesel production from microalgae in ponds. Biore Technol 102:50–56
Carpenter JH (1966) New measurements of oxygen solubility in pure and natural waters. Limnol Oceanogr 11:264–277
Cosgrove J, Borowitzka MA (2006) Applying pulse amplitude modulation (PAM) fluorometry to microalgae suspensions: stirring potentially impacts fluorescence. Photosyn Res 88:343–350
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187
Diesendorf M (2003), Australia's polluting power: coal-fired electricity and its impact on global warming, WWF Australia, Sydney. (www.wwf.org.au). Accessed: 8 January 2012
Energy Information Administration (2008) Annual energy outlook. Department of Energy, Washington DC
Fon Sing S, Isdepsky A, Borowitzka MA, Moheimani NR (2011) Production of biofuels from microalgae. Mitig Adapt Strat Global Change. doi:10.1007/s11027-011-9294-x
Geider RJ, Delucia EH, Falkowski P, Finzi A, Grime PJ, KanaTM RJ, Long SP, Osborne BAD (2001) Primary productivity of planet earth: biological determinants and physical constraints in terrestrial and aquatic habitats. Global Change Biol 7:849–882
González-López CV, Acién Fernández FG, Fernández-Sevilla JM, Sánchez Fernández JF, Molina Grima E (2012) Development of a process for efficient use of CO2 from flue gases in the production of photosynthetic microorganisms. Biotech Bioeng. doi:10.1002/bit.24446
Grobbelaar J (1994) Turbulence in mass algal cultures and the role of light/dark fluctuations. JAppl Phycol 6:331–335
Herzog HJ, Drake EM (1996) Carbon dioxide recovery and disposal from large energy systems. Ann Rev Energ Environ 21:145–166
Hogetsu D, Miyachi S (1979) Role of carbonic anhydrase in photosynthetic CO2 fixation in Chlorella. Plant Cell Physiol 20:747–756
Hooper B, Innocenzi T, Dugan C (2009) The hazelwood/H3 cature demonstration projects In: International Pittsburgh Coal Conference Pittsburgh, PA. Curran Associates, Inc, New York
Hughes E, Benemann J (1997) Biological fossil CO2 mitigation. Energ Conv Manag 38:S467–S473
Kawachi M, Noel M (2005) Sterilization and sterile techniques. In: Andersen RA (ed) Algal culturing technique. Elsevier, London, pp 65–81
Krause GH, Weis E (1984) Chlorophyll fluorescence as a tool in plant physiology. II. Interpretation of fluorescence signals. Photos Res 5:139–157
Lewis ER, Wallace DWR (1998) Program developed for CO2 system calculations. BNL-61827 informal. Oak Ridge National Laboratory, Oak Ridge
Maeda K, Owada M, Kimura N, Omata K, Karube I (1995) CO2 fixation from the flue gas on coal-fired thermal power plant by microalgae. Energ Conv Manag 36:717–720
Mercz TI (1994) A study of high lipid yielding microalgae with potential for large-scale production of lipids and polyunsaturated fatty acids. PhD Thesis, School of Biological Sciences and Biotechnology. Murdoch University, Perth, p 278
Millero FJ (1979) The thermodynamics of the carbonate system in seawater. Geochim Cosmochim Acta 43:1651–1661
Moheimani NR (2012) Long term outdoor growth and lipid productivity of fresh and seawater green microalgae (Chlorophyta) in bag photobioreactors. J Appl Phycol. doi:10.1007/s10811-012-9850-0
Moheimani NR, Borowitzka MA (2006a) Limits to productivity of the alga Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. Biotech Bioeng 96:27–36
Moheimani NR, Borowitzka MA (2006b) The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J Appl Phycol 10:703–712
Moheimani NR, Borowitzka MA (2011) Increased CO2 and the effect of pH on growth and calcification of Pleurochrysis carterae and Emiliania huxleyi (Haptophyta) in semicontinuous cultures. Appl Microbiol Biot 90(4):1399–1407
Moheimani NR, Isdepsky A, Lisec J, Raes E, Borowitzka MA (2011) Coccolithophorid algae culture in closed photobioreactors. Biotech Bioeng 108:2078–2087
Muller-Feuga A, Robert R, Cahu C, RobinJ DP (2003) Uses of microalgae in aquaculture. In: Stottrup JG, McEvoy LA (eds) Live Feeds in Marine Aquaculture. Blackwell, Oxford, pp 253–299
Negoro M, Shioji N, Miyamoto K, Miura Y (1991) Growth of microalgae in high CO2 gas and effects of SOx and NOx. Appl Biochem Biotech 28–9:877–886
Negoro M, Shioji N, Ikuta Y, Makita T, Uchiumi M (1992) Growth characteristics of microalgae in high-concentration CO2 gas. Effects of culture medium trace components, and impurities thereon. Appl Biochem Biotech 34–5:681–692
Negoro M, Hamasaki A, Ikuta Y, Makita T, Hirayama K, Suzuki S (1993) Carbon dioxide fixation by microalgae photosynthesis using actual flue gas discharged from a boiler. Appl Biochem Biotech 39:643–653
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotech 65:635–648
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Raven JA (1997) Putting the C in phycology. Europ J Phycol 32:319–333
Rigobello-Masini M, Aidar E, Masini JC (2003) Extra and intracelular activities of carbonic anhydrase of the marine microalga Tetraselmis gracilis (Chlorophyta). Brazil J Microbiol 34:267–272
Roy RN, Roy LN, Vogel KM, Porter-Moore C, Pearson T, Good CE, Millero FJ, Campbell DM (1993) The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45 °C. Mar Chem 44:249–267
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis, 2nd edn. Fisheries Research Board of Canada Bulletin 167. Fish Res Board Canada, Ottawa
Vitousek PM (1994) Beyond global warming: ecology and global change. Ecol 75:1861–1876
Acknowledgment
This study was solely funded by Victor Smorgon Group and with collaboration with the IPH. I would especially like to thank Mr Peter Edwards and Mr Jonathon Green of the Victor Smorgon Group for their unlimited support. I also greatly appreciate the unlimited support received from Tony Innocenzi, Chris Barfoot and Peter Massey from IPH. Especial thanks to Chris Barfoot for his assistance with the outdoor cultivation and laboratory experiments. Also thanks to Sam Muresan, Dale Newing and Peter Gestos for managing the outdoor bag photobioreactors. Also thanks to the colleagues from BioMax for their unlimited support in setting up and conducting these experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moheimani, N.R. Inorganic carbon and pH effect on growth and lipid productivity of Tetraselmis suecica and Chlorella sp (Chlorophyta) grown outdoors in bag photobioreactors. J Appl Phycol 25, 387–398 (2013). https://doi.org/10.1007/s10811-012-9873-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9873-6