Abstract
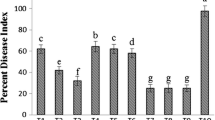
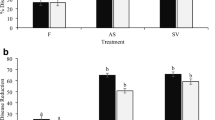
This study examined the effects of Stimplex™, a marine plant extract formulation from Ascophyllum nodosum, on some common cucumber fungal pathogens. Greenhouse cucumber plants were sprayed and/or root drenched using Stimplex™ at 0.5% or 1% concentration twice at 10-day intervals. Treatments also included application of fungicide (chlorothalonil, 2 g L−1) alternating with Stimplex™ application. Treated plants were inoculated with four cucumber fungal pathogens including Alternaria cucumerinum, Didymella applanata, Fusarium oxysporum, and Botrytis cinerea. Stimplex™ application resulted in a significant reduction in disease incidence of all the pathogens tested. The disease control effect was greater for Alternaria and Fusarium infection, followed by Didymella and Botrytis. Combined spray and root drenching with Stimplex™ was more effective than either spray or root drenching alone. The alternation of one fungicide application, alternated with Stimplex™ application, was highly effective and found to be the best treatment in reducing the disease ratings. Plants treated with Stimplex™ showed enhanced activities of various defense-related enzymes including chitinase, β-1,3-glucanase, peroxidase, polyphenol oxidase, phenylalanine ammonia lyase, and lipoxygenase. Altered transcript levels of various defense genes, including chitinase, lipoxygenase, glucanase, peroxidase, and phenylalanine ammonia lyase were observed in treated plants. Cucumber plants treated with Stimplex™ also accumulated higher level of phenolics compared to water controls. These results suggest that seaweed extracts enhance disease resistance in cucumber probably through induction of defense genes or enzymes.





Similar content being viewed by others
References
Alexrod B, Cheesbrough TM, Laakso S (1981) Lipoxygenases in soybean. Meth Enzymol 71:441–451
Anderson AJ, Blee KA, Yang KY (2006) Commercialization of plant systemic defense activation: theory, problems and successes. In: Tuzun T, Bent E (eds) Multigenic and induced systemic resistance in plants. Springer, New York, pp 386–414
Andreas W, Oliver S (2009) β-Aminobutyric acid-induced resistance in cucumber against biotrophic and necrotrophic pathogens. J Phytopathol 157:356–361
Benhamou N, Kloepper JW, Tuzun S (1994) Induction of systemic resistance to Fusarium wilt of tomato plants by seed treatment with chitosan. Phytopathology 84:1432–1444
Cluzet S, Torregrosa C, Jacquet C, Lafitte C, Fournier J, Mercier L, Salamagne S, Briand X, Esquerre-Tugaye MT, Dumas B (2004) Gene expressing profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green alga Ulva spp. Plant Cell Environ 27:917–928
Colapietra M, Alexander A (2006) Effect of foliar fertilization on yield and quality of table grapes. Acta Hortic (ISHS) 721:213–218
Ganesan V, Thomas G (2001) Salicylic acid response in rice: influence of salicylic acid on H2O2 accumulation and oxidative stress. Plant Sci 160:1095–1106
Garcia-Mina JM, Goicoechea N, Aguirreolea J (2004) Alleviation of Verticillium wilt in pepper (Capsicum annuum L) by using the organic amendment COA H of natural origin. Sci Hortic 101:23–37
Hammerschmidt R (1999) Induced disease resistance: how do induced plants stop pathogens? Physiol Mol Plant Pathol 55:77–84
Hukkanen AT, Kokko HI, Buchala AJ, McDougall GJ, Stewart D, Kärenlampi SO, Karjalainen RO (2007) Benzothiadiazole induces the accumulation of phenolics and improves resistance to powdery mildew in strawberries. J Agric Food Chem 55:1862–1870
Jayaraj J, Punja ZK (2007) Combined expression of chitinase and lipid transfer protein genes in transgenic carrot plants enhances resistance to foliar fungal pathogens. Plant Cell Rep 26:1539–1546
Jayaraj J, Anand A, Muthukrishnan S (2004) Pathogenesis-related proteins and their roles in resistance to fungal pathogens. In: Punja ZK (ed) Fungal disease resistance in plants—biochemistry, molecular biology and genetic engineering. Food Products Press, New York, pp 139–178
Jayaraj J, Wan A, Rahman M, Punja ZK (2008) Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot 10:1360–1366
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges M, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Klarzynski O, Plesse B, Joubert JM, Yvin JC, Kopp M, Kloareg B, Fritig B (2000) Linear ß-1, 3 glucans are elicitors of defence responses in tobacco. Plant Physiol 124:1027–1038
Kobayashi A, Tai A, Kanzaki H, Kawazu K (1993) Elicitor-active oligosaccharides from algal laminarin stimulate the production of antifungal compounds in alfalfa. Z Naturforsch 48c:575–579
Kombrink E, Somssich IE (1995) Defense responses of plants to pathogens. In: Andrews JH, Tommerup IC (eds) Advances in botanical research, vol 21. Academic, New York, pp 1–34
Lin TC, Ishizaka M, Ishii H (2008) Acibenzolar-S-methyl-induced systemic resistance against anthracnose and powdery mildew diseases on cucumber plants without accumulation of phytoalexins. J Phytopathol 157:40–50
Lizzi Y, Coulomb C, Polian C, Coulomb PJ, Coulomb PO (1998) Seaweed and mildew: what does the future hold? Encouraging laboratory results. Phytoma 508:29–30
Mercier L, Lafitte C, Borderies G, Briand X, Esquerre-Tugaye MT, Fournier J (2001) The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol 149:43–51
Norrie J, Branson T, Keathley PE (2002) Marine plants extracts impact on grape yield and quality. Acta Hort (ISHS) 594:315–319
Ohta H, Shida K, Peng YL, Furasawa I, Shishiyama J, Aibara S, Morita Y (1990) The occurrence of lipid hydroperoxide decomposing-enzyme activities in rice and the relationship of such activities to the formation of antifungal substances. Plant Cell Physiol 31:1117–1122
Peng X, Zhang H, Bai Z, Li B (2004) Induced resistance to Cladosporium cucumerinum in cucumber by pectinases extracted from Penicillium oxalicum. Phytoparasitica 32:377–387
Postma J, Willemsen-de Klein MJEIM, van Elsas JD (2000) Effect of the indigenous microflora on the development of root and crown rot caused by Pythium aphanidermatum in cucumber grown on rockwool. Phytopathology 90:125–133
Rahman M, Punja ZK (2005) Biochemistry of ginseng root tissues affected by rusty root symptoms. Plant Physiol Biochem 43:1103–1114
Rathore SS, Chaudhary DR, Boricha GN, Ghosh A, Bhatt BP, Zodape ST, Patolia JS (2009) Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S Afr J Bot 75:351–355
Rose S, Parker M, Punja ZK (2003) Efficacy of biological and chemical treatments for control of Fusarium root and stem rot on greenhouse cucumber. Plant Dis 87:1462–1470
Ryan CA, Farmer EE (1991) Oligosaccharide signals in plants: a current assessment. Annu Rev Plant Physiol Plant Mol Biol 42:651–674
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89:92–99
Sutherland MW (1991) The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol 39:79–93
Ton J, Corne MJ, Pieterse D, van Loon LC (2006) The relationship between basal and induced resistance in Arabidopsis. In: Tuzun T, Bent E (eds) Multigenic and induced systemic resistance in plants. Springer, NY, USA, pp 197–225
Trotel-Aziz K, Couderchet M, Vernet G, Aziz A (2006) Chitosan stimulates defense reactions in grapevine leaves and inhibits development of Botrytis cinerea. Eur J Plant Pathol 114:405–413
Vallard GE, Goodman RM (2004) Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci 44:1920–1934
Vander P, Kjell MV, Domard A, El-Gueddari NE, Moerschbacher BM (1998) Comparison of the ability of partially N-acetylated chitosans and oligosaccharides to elicit resistance in wheat leaves. Plant Physiol 118:1353–1359
Vasuikova SV, Zinoveva LI, Ilinskaia EA, Perekhod NG, Chalenko GI, Gerasimova AV, Iiina P, Varlamov V, Zeretskovskia K (2001) Modulation of plant disease by water-soluble chitosan. Prikl Biokhim Mikrobiol 37:115–122
Vidhyasekaran P (1997) Fungal pathogenesis in plants and crops: molecular biology and host defense mechanisms. Marcel Decker, New York
Walters D, Walsh D, Newton A, Lyon G (2005) Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology 95:1368–1373
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Meth Enzymol 160:87–112
Zuniga TL, Jantz JP, Zitter TA, Jahn MK (1999) Monogenic dominant resistance to gummy stem blight in two melon (Cucumis melo) accessions. Plant Dis 83:1105–1107
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayaraman, J., Norrie, J. & Punja, Z.K. Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J Appl Phycol 23, 353–361 (2011). https://doi.org/10.1007/s10811-010-9547-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9547-1