Abstract
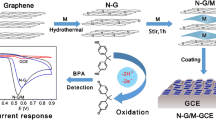
Due to the unique electronic properties and high adsorption capacity of graphene, a facile strategy was developed to form graphene/melamine nanocomposite by anchoring melamine molecule on the surface of graphene sheets. As the electrostatic attraction happened between protonated melamine and negatively charged bisphenol A (BPA), a novel electrochemical sensor was fabricated to determine the endocrine disruptor BPA by depositing graphene/melamine nanocomposite on the surface of glassy carbon electrode. The electrochemical behavior of BPA was investigated in phosphate buffer solution (pH 7.0) using the prepared sensor. A well-defined anodic peak at 0.56 V was found to attribute to the electrooxidation of BPA on the modified electrode. The kinetic parameters, charge transfer coefficient, electron transfer number, proton transfer number, and standard rate constant were calculated and optimized. The electrochemical sensor exhibited a wider linear range of 1.0 × 10−8 to 2.0 × 10−4 M BPA and a lower detection limit of 4.0 × 10−9 M (S/N = 3). This novel sensor was successfully applied to determine BPA leached from real plastic samples with good recoveries ranging from 97.00 to 100.96 %.








Similar content being viewed by others
References
Alkasir RS, Ganesana M, Won YH, Stanciu L, Andreescu S (2010) Enzyme functionalized nanoparticles for electrochemical biosensors: a comparative study with applications for the detection of bisphenol A. Biosens Bioelectron 26:43–49. doi:10.1016/j.bios.2010.05.001
Ballesteros-Gómez A, Rubio S, Pérez-Bendito D (2009) Analytical methods for the determination of bisphenol A in food. J Chromatogr A 1216:449–469. doi:10.1016/j.chroma.2008.06.037
Segner H, Caroll K, Fenske M, Janssen CR, Maack G, Pascoe D, Chafers C, Vandenbergh GF, Watts M, Wenzel A (2003) Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: report from the European IDEA project. Ecotoxicol Environ Saf 54:302–314. doi:10.1016/S0147-6513(02)00039-8
Soto AM, Sonnenschein C (2010) Environmental causes of cancer: endocrine disruptors as carcinogens. Nat Rev Endocrinol 6:363–370. doi:10.1038/nrendo.2010.87
Chang CM, Chou CC, Lee MR (2005) Determining leaching of bisphenol A from plastic containers by solid-phase microextraction and gas chromatography–mass spectrometry. Anal Chim Acta 539:41–47. doi:10.1016/j.aca.2005.03.051
Nerin C, Philo MR, Salafranca J, Castle L (2002) Determination of bisphenol-type contaminants from food packaging materials in aqueous foods by solid-phase microextraction-high-performance liquid chromatography. J Chromatogr A 963:375–380. doi:10.1016/S0021-9673(02)00554-X
Kuramitz H, Matsushita M, Tanaka S (2004) Electrochemical removal of bisphenol A based on the anodic polymerization using a column type carbon fiber electrode. Water Res 38:2331–2338. doi:10.1016/j.watres.2004.02.023
Yin H, Zhou Y, Ai S, Han R, Tang T, Zhu L (2010) Electrochemical behavior of bisphenol A at glassy carbon electrode modified with gold nanoparticles, silk fibroin, and PAMAM dendrimers. Microchim Acta 170:99–105. doi:10.1007/s00604-010-0396-z
Chauke V, Matemadombo F, Nyokong T (2010) Remarkable sensitivity for detection of bisphenol A on a gold electrode modified with nickel tetraamino phthalocyanine containing Ni–O–Ni bridges. J Hazard Mater 178:180–186. doi:10.1016/j.jhazmat.2010.01.061
Zhu J, Chen S, Zhou H, Wang X (2012) Fabrication of a low defect density graphene-nickel hydroxide nanosheet hybrid with enhanced electrochemical performance. Nano Res 5:11–19. doi:10.1007/s12274-011-0179-9
Yuan J, Zhu J, Bi H, Meng X, Liang S, Zhang L, Wang X (2013) Graphene-based 3D composite hydrogel by anchoring Co3O4 nanoparticles with enhanced electrochemical properties. Phys Chem Chem Phys 15:12940–12945. doi:10.1039/C3CP51710A
Cai YY, Li H, Du B, Yang MH, Li Y, Wu D, Zhao YF, Dai YX, Wei Q (2011) Ultrasensitive electrochemical immunoassay for BRCA1 using BMIM BF4-coated SBA-15 as labels and functionalized graphene as enhancer. Biomaterials 32:2117–2123
Lu CH, Yang HH, Zhu CL, Chen X, Chen GN (2009) A graphene platform for sensing biomolecules. Angew Chem 121:4879–4881. doi:10.1002/ange.200901479
Ao ZM, Peeters FM (2010) Electric field activated hydrogen dissociative adsorption to nitrogen-doped graphene. J Phys Chem C 114:14503–14509. doi:10.1021/jp103835k
Ntsendwana B, Mamba BB, Sampath S, Arotiba OA (2012) Electrochemical detection of bisphenol A using graphene-modified glassy carbon electrode. Int J Electrochem Sci 7:3501–3512
Wang Q, Wang Y, Liu S, Wang L, Gao F, Gao F, Sun W (2012) Voltammetric detection of bisphenol a by a chitosan–graphene composite modified carbon ionic liquid electrode. Thin Solid Films 520:4459–4464. doi:10.1016/j.tsf.2012.02.069
Lu S, Fei J, He Q, Hu S (2004) Application of a multi-wall carbon nanotubes film coated electrode for the determination of bisphenol A leached from plastic waste samples. Chem Anal 49:607–617
Yu C, Gou L, Zhou X, Bao N, Gu H (2011) Chitosan–Fe3O4 nanocomposite based electrochemical sensors for the determination of bisphenol A. Electrochim Acta 56:9056–9063. doi:10.1016/j.electacta.2011.05.135
Yin H, Cui L, Chen Q, Shi W, Ai S, Zhu L, Lu L (2011) Amperometric determination of bisphenol A in milk using PAMAM–Fe3O4 modified glassy carbon electrode. Food Chem 125:1097–1103. doi:10.1016/j.foodchem.2010.09.098
Liu X, Feng H, Liu X, Wong DK (2011) Electrocatalytic detection of phenolic estrogenic compounds at NiTPPS/carbon nanotube composite electrodes. Anal Chim Acta 689:212–218. doi:10.1016/j.aca.2011.01.037
Li Q, Li H, Du GF, Xu ZH (2010) Electrochemical detection of bisphenol A mediated by [Ru(bpy)3]2+ on an ITO electrode. J Hazard Mater 180:703–709. doi:10.1016/j.jhazmat.2010.04.094
Andreescu S, Sadik OA (2004) Correlation of analyte structures with biosensor responses using the detection of phenolic estrogens as a model. Anal Chem 76:552–560. doi:10.1021/ac034480z
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1342. doi:10.1021/ja01539a017
He G, Chen H, Zhu J, Bei F, Sun X, Wang X (2011) Synthesis and characterization of graphene paper with controllable properties via chemical reduction. J Mater Chem 21:14631–14638. doi:10.1039/C1JM12393A
Kuramitz H, Nakata Y, Kawasaki M, Tanaka S (2001) Electrochemical oxidation of bisphenol A. Application to the removal of bisphenol A using a carbon fiber electrode. Chemosphere 45(1):37–43. doi:10.1016/S0045-6535(01)00032-7
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Cao Q, Zhao H, Zeng L, Wang J, Wang R, Qiu X, He Y (2009) Electrochemical determination of melamine using oligonucleotides modified gold electrodes. Talanta 80:484–488
Yin H, Zhou Y, Xu J, Ai S, Cui L, Zhu L (2010) Amperometric biosensor based on tyrosinase immobilized onto multiwalled carbon nanotubes-cobalt phthalocyanine-silk fibroin film and its application to determine bisphenol A. Anal Chim Acta 659:144–150. doi:10.1016/j.aca.2009.11.051
Laviron E (1974) Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 52:355–393. doi:10.1016/S0022-0728(74)80448-1
Velasco JG (1997) Determination of standard rate constants for electrochemical irreversible processes from linear sweep voltammograms. Electroanalysis 9:880–882. doi:10.1002/elan.1140091116
Gooding JJ, Praig VG, Hall EAH (1998) Platinum-catalyzed enzyme electrodes immobilized on gold using self-assembled layers. Anal Chem 70(11):2396–2402. doi:10.1021/ac971035t
Anson FC (1964) Application of potentiostatic current integration to the study of the adsorption of cobalt (III)-(ethylenedinitrilo) tetraacetate on mercury electrodes. Anal Chem 36:932–934. doi:10.1021/ac60210a068
Velasco JG (1997) Determination of standard rate constants for electrochemical irreversible processes from linear sweep voltammograms. Electroanalysis 9(11):880–882. doi:10.1002/elan.1140091116
Acknowledgments
This study was funded by the National Natural Science Foundation of China (No. 51202020, 51472035), the Science and Technology Department of Jiangsu Province (BY2012099, BY2013024-04, BE2014089), Jiangsu Key Lab of Advanced Catalytic Materials and Technology (BM2012110), and the Qing Lan Project of Jiangsu Province of China.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shen, R., Zhang, W., Yuan, Y. et al. Electrochemical detection of bisphenol A at graphene/melamine nanoparticle-modified glassy carbon electrode. J Appl Electrochem 45, 343–352 (2015). https://doi.org/10.1007/s10800-015-0792-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0792-5