Abstract
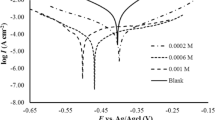
EIS was utilized as a novel approach to study the role of mechanical and electrochemical processes in flow accelerated pitting corrosion behaviour of AA5083-H321 aluminum–magnesium alloy in 3.5% NaCl solution. This alloy is a suitable material for manufacturing of high speed boats, submarines, desalination systems etc. Impedance spectra were obtained during 24 h of exposure of the samples to the test solution at different rotation speeds. The surface and cross section of the samples were studied by scanning electron microscopy (SEM) and EDAX analysis. The results indicated that increasing the rotation speed causes the depth of pits to increase. By further increasing the rotation speed to 5 and 7 m s−1, the flow condition causes the passive layer inside the pits to breakdown. Simultaneously, the thickness of the passive layer on the areas other than the pits becomes thinner. Shear stresses at 10 m s−1 are so severe that the passive layer on the entire surface breaks down and leads to micropitting corrosion.








Similar content being viewed by others
References
Chester HH (1985) Mar Technol 22:155
Brown S (1994) Feasibility of replacing structural steel with aluminum alloy s in shipbuilding industry. Report Published by University of Viscontin at Madison
Davis JR (1999) Corrosion of aluminum and aluminum alloys. ASM International, Materials Park, OH
Bethencourt M, Botana FJ (1998) Mater Sci Forum 289–292:567
Barbucci A, Bruzzone G (2000) Intermetallics 8:305
Birbilis N, Buchheit RG (2005) J Electrochem Soc 152:140
Nisancioglu K (2004) Corrosion and protection of aluminum alloys in seawater, Eurocorr 2004
Ghering GA, Peterson MH (1981) Corrosion 37:232
Davis JA, Ghering GA (1975) Mater Perform 4:87
Jafarzadeh K, Shahrabi T, Hosseini MG et al (2007) J Mater Sci Technol 23:623
Mansfeld F (1990) Electrochim Acta 35:1533
Macdonald DD (2006) Electrochim Acta 51:1376
Silverman DC, Carrico JE (1988) Corrosion 44:280
Aballe A, Bethencourt M, Botana FJ (2001) Mater Corros 53:185
SSPC Standards, systems and specifications (1995) Edited by Janes Rex 112
De Wit JH, Lenderink HJW (1996) Electrochim Acta 41:1111
Smialowska ZS (1999) Corros Sci 41:1743
Aballe A, Bethencourt M, Botana FJ et al (2001) Corros Sci 43:1657
Aballe A, Bethencourt M, Botana FJ et al (2003) Corros Sci 45:161
Frers SE, Stefenel MM, Mayer C et al (1990) J Appl Electrochem 20:996
Jafarzadeh K, Shahrabi T, Hosseini MG et al (2008) J Mater Sci Technol 24:215
http://www.electronics-tutorials.com/basics/Inductance (2007)
Juttner K (1990) Electrochim Acta 35:1501
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jafarzadeh, K., Shahrabi, T. & Oskouei, A.A. Novel approach using EIS to study flow accelerated pitting corrosion of AA5083-H321 aluminum–magnesium alloy in NaCl solution. J Appl Electrochem 39, 1725–1731 (2009). https://doi.org/10.1007/s10800-009-9867-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9867-5