Abstract
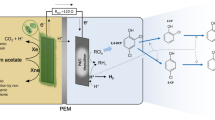
This article presents a mathematical model that predicts the chemical conditions at the electrode surface during the electrochemical reduction of CO2. Such electrochemical reduction of CO2 to valuable products is an area of interest for the purpose of reducing green house gas emissions. In the reactions involved, CO2 acts as both a reactant and a buffer, consequently the estimation of local concentrations at the electrode surface is not trivial and a numerical approach is required. The necessary partial differential equations (PDEs) have been set-up and solved using MATLAB. The results show the local concentrations at the electrode surface to be significantly different from the bulk concentrations under typical reported experimental conditions. The importance of buffer strength and a careful quantification of the degree of mixing produced in the experimental apparatus is demonstrated. The model has also been used to re-examine previously published data, showing that the Tafel slopes in CO2 reduction are consistent with those reported for the simpler CO reduction system. Further, the effect of pulsed electroreduction was also modeled, showing that pulsing causes corresponding swings in local pH and CO2 concentrations.
Similar content being viewed by others
Abbreviations
- Symbol :
-
Description (Units)
- cef\(_{{\rm CH}_{4}}\) :
-
current efficiency for methane formation (dimensionless)
- cef\(_{{\rm C}_{2}{\rm H}_{4}}\) :
-
Current efficiency for ethylene formation (dimensionless)
- cefCO :
-
current efficiency for carbon monoxide formation (dimensionless)
- cef\(_{{\rm H}_{2}}\) :
-
current efficiency for hydrogen formation (dimensionless)
- cef\(_{{\rm HCOO}^{-}}\) :
-
current efficiency for formate formation (dimensionless)
- CO2 consumption :
-
rate of CO2 consumption at cathode surface (kmol m−2 s−1)
- D \(^{0}_{{\rm CO}_{2}}\) :
-
diffusion coefficient for carbon dioxide in water at 25 °C at infinite dilution (m2 s−1)
- D \(^{0}_{{\rm CO}_{3}^{-}}\) :
-
diffusion coefficient for carbonate ions in water at 25 °C at infinite dilution (m2 s−1)
- D \(^{0}_{{\rm HCO}_{3}^{-}}\) :
-
diffusion coefficient for bicarbonate ions in water at 25 °C at infinite dilution (m2 s−1)
- D \(^{0}_{{\rm OH}^{-}}\) :
-
diffusion coefficient for hydroxyl ions at 25 °C at infinite dilution (m2 s−1)
- D \(_{{\rm CO}_{2}}\) :
-
diffusion coefficient for carbon dioxide in water at 25 °C and given electrolyte concentration (m2 s−1)
- D \(_{{\rm CO}_{3}^{-}}\) :
-
diffusion coefficient for carbonate ions in water at 25 °C and given electrolyte concentration (m2 s−1)
- D HCO −3 :
-
diffusion coefficient for bicarbonate ions in water at 25 °C and given electrolyte concentration (m2 s−1)
- D \(_{{\rm OH}^{-}}\) :
-
diffusion coefficient for hydroxyl ions at 25 °C and given electrolyte concentration (m2 s−1)
- F :
-
Faraday’s constant (96486) (C mol−1)
- j :
-
current density at the Cu electrode (A m−2)
- k 1f :
-
rate constant for forward reaction (3b) (M−1 s−1)
- k 1r :
-
rate constant for reverse reaction (3b) (M−1 s−1)
- k 2f :
-
rate constant for forward reaction (4) (s−1)
- k 2r :
-
rate constant for reverse reaction (4) (s−1)
- K H :
-
equilibrium constant for reaction (1) (dimensionless)
- K 1a :
-
equilibrium constant for reaction (3a) (M)
- K 1b :
-
equilibrium constant for reaction (3b) (M−1)
- K 2 :
-
equilibrium constant for reaction (4) (M−1)
- K 3 :
-
equilibrium constant for reaction (5) (dimensionless)
- OH\(^{-}_{\rm formation}\) :
-
rate of OH− formation at cathode surface (kmol m−2 s−1)
- zeff\(_{{\rm CH}_{4}}\) :
-
electrons exchanged in reaction (13) (dimensionless)
- zeff\(_{{\rm C}_{2}{\rm H}_{4}}\) :
-
electrons exchanged in reaction (14) (dimensionless)
- zeffCO :
-
electrons exchanged in reaction (12) (dimensionless)
- zeffH 2 :
-
electrons exchanged in reaction (15) (dimensionless)
- zeff\(_{{\rm HCOO}^{-}}\) :
-
electrons exchanged in reaction (11) (dimensionless)
- Greek :
-
Description (Units)
- δ:
-
boundary layer thickness (m)
- μ:
-
viscosity of electrolyte solution (mPa s or cP)
References
Bandi A., Specht M., Weimer T., Schaber K. (1995) Energy Conversion Management 36:899
Tryk D.A., Fujishima A. (2001) The Electrochem. Soc. Interface 10:32
Sullivan B.P., Krist K., Guard H.E. (eds). (1993). Electrochemical and Electrocatalytic Reactions of Carbon Dioxide. Elsevier, Amsterdam
Y. Hori, in W. Vielstich and H.A. Gasteiger and A. Lamm (Eds), Handbook of Fuel Cells’, (John Wiley and Sons, Ltd., West Sussex, England 2 2003) p. 720
Teeter T.E., Van Rysselberghe P. (1954) J. Chem. Phys 22:759
Ito K., Murata T., Ikeda S. (1975) Bull. Nagoya Inst. Techn 27:209
Stevens G.B., Reda T., Raguse B. (2002) J. Electroanal. Chem 526:125
Y. Hori, K. Kikuchi and S. Suzuki, Chem. Lett. (1985) 1695
Hori Y., Akira M., Takahashi R. (1989) J. Chem. Soc. Faraday Trans. 1 85:2309
Y. Hori, K. Kikuchi, A. Murata and S. Suzuki, Chem. Lett. (1986) 897
D.R. Lide, CRC Handbook of Chemistry and Physics, Internet Version 2005, <http://www.hbcpnetbase.com>, (CRC Press, Boca Raton, FL, 2005)
Hori Y., Takahashi R., Yoshinami Y., Murata A. (1997) J. Phys. Chem. B 101:7075
Hori Y., Akira M., Yoshinami Y. (1991) J. Chem. Soc. Faraday Trans. 1 87:125
Cook R.L., MacDuff R.C., Sammells A.F. (1988) Journal of the Electrochem. Soc 135:1320
Smith B.D., Irish D.E., Kedzierzawski P., Augustynski J. (1997) J. Electrochem. Soc 144:4288
Shiratsuchi R., Aikoh Y., Nogami G. (1993) J. Electrochem. Soc 140:3479
Jermann B., Augustynski J. (1994). Electrochimica Acta 39:1891
Acknowledgements
The authors would like to thank the Innovative Research Initiative for Greenhouse Gas Mitigation for their financial support for this work, and Prof. Colin Oloman of the University of British Columbia for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, N., Gattrell, M. & MacDougall, B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J Appl Electrochem 36, 161–172 (2006). https://doi.org/10.1007/s10800-005-9058-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-005-9058-y